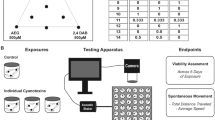
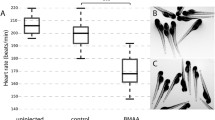
Abstract
β-N-Methylamino-l-alanine (BMAA) is a non-proteinogenic amino acid produced by cyanobacteria, which has been implicated in several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). It is postulated that chronic exposure to BMAA can lead to formation of protein aggregates, oxidative stress, and/or excitotoxicity, which are mechanisms involved in the etiology of ALS. While specific genetic mutations are identified in some instances of ALS, it is likely that a combination of genetic and environmental factors, such as exposure to the neurotoxin BMAA, contributes to disease. We used a transgenic zebrafish with an ALS-associated mutation, compared with wild-type fish to explore the potential neurotoxic effects of BMAA through chronic long-term exposures. While our results revealed low concentrations of BMAA in the brains of exposed fish, we found no evidence of decreased swim performance or behavioral differences that might be reflective of neurodegenerative disease. Further research is needed to determine if chronic BMAA exposure in adult zebrafish is a suitable model to study neurodegenerative disease initiation and/or progression.






Similar content being viewed by others
Availability of Data and Materials
Contact corresponding author for access to data and materials.
Change history
24 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12640-023-00664-1
References
Ackermann GE, Paw BH (2003) Zebrafish: a genetic model for vertebrate organogenesis and human disorders. Front Biosci 8:1227–1253
Andersson M, Karlsson O, Bergström U, Brittebo EB, Brandt I (2013) Maternal Transfer of the Cyanobacterial Neurotoxin β-N-Methylamino-L-Alanine (BMAA) via Milk to Suckling Offspring. PLoS One 8
Andersson M, Karlsson O, Brandt I (2018) The environmental neurotoxin β-N-methylamino-L-alanine (L-BMAA) is deposited into birds’ eggs. Ecotoxicol Environ Saf 147:720–724
Babin PJ, Goizet C, Raldúa D (2014) Zebrafish models of human motor neuron diseases: Advantages and limitations. Prog Neurobiol 118:36–58
Banack SA (2021) Second Laboratory Validation of β-N-Methylamino-L-Alanine, N-(2aminoethyl)Glycine, and 2,4-Diaminobuytric Acid by Ultra-Performance Liquid Chromatography and Tandem Mass Spectrometry. Neurotox Res 39:107–116
Banack SA, Murch SJ, Cox PA (2006) Neurotoxic flying foxes as dietary items for the Chamorro people. Marianas Islands J Ethnopharmacol 106:97–104
Baumgart F, Rodríguez-Crespo I (2008) D-Amino acids in the brain: the biochemistry of brain serine racemase. FEBS J 275:3538–3545
Beri J, Nash T, Martin RM, Bereman MS (2017) Exposure to BMAA mirrors molecular processes linked to neurodegenerative disease. Proteomics 17:17–18
Brand LE, Pablo J, Compton A, Hammerschlag N, Mash DC (2010) Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 9:620–635
Cachat J et al (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5:1786–1799
Caller TA et al (2009) A cluster of amyotrophic lateral sclerosis in New Hampshire: a possible role for toxic cyanobacteria blooms. Amyotroph Lateral Scler 10:101–108
Caller T, Henegan P, Stommel E (2018) The Potential Role of BMAA in Neurodegeneration. 222–226. https://doi.org/10.1007/s12640-017-9752-7
Chiu AS et al (2012) Excitotoxic potential of the cyanotoxin β-methyl-amino-l-alanine (BMAA) in primary human neurons. Toxicon 60:1159–1165
Chiu AS, Gehringer MM, Welch JH, Neilan BA (2011) Does alpha-amino-beta-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int J Environ Res Public Health 8:3728–3746
Cohen SA (2012) Analytical techniques for the detection of a-amino-b-methylaminopropionic acid. Analyst 137:1991–2005
Cox PA et al (2009) Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph Lateral Scler 10:109–117
Cox PA, Banack SA, Murch SJ (2003) Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci 100:13380–13383
Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA (2016) Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc r Soc B Biol Sci 283:1–10
Davis DA et al (2019) Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 14:1–18
Davis DA et al (2020) L -Serine Reduces Spinal Cord Pathology in a Vervet Model of Preclinical ALS / MND. J Neuropathol Exp Neurol 1–14
Duncan MW et al (1991) 2-Amino-3-(Methylamino)-Propanoic Acid (BMAA) Pharmacokinetics and Blood-Brain Barrier Permeability in the Rat. J Pharmacol Exp Ther 258:27–35
Dunlop RA, Cox PA, Banack SA, Rodgers KJ (2013) The Non-Protein Amino Acid BMAA Is Misincorporated into Human Proteins in Place of l-Serine Causing Protein Misfolding and Aggregation. PLoS One 8
Dunlop DS, Neidle A (2005) Regulation of serine racemase activity by amino acids. Mol Brain Res 133:208–214
Eichler EE (2019) Genetic Variation, Comparative Genomics, and the Diagnosis of Disease. N Engl J Med 381:64–74
Engskog MKR et al (2013) The cyanobacterial amino acid β-N-methylamino-l-alanine perturbs the intermediary metabolism in neonatal rats. Toxicology 312:6–11
Frøyset AK, Khan EA, Fladmark KE (2016) Quantitative proteomics analysis of zebrafish exposed to sub-lethal dosages of β-methyl-amino-L-alanine (BMAA). Sci Rep 6:29631
Jiang L, Kiselova N, Rosén J, Ilag LL (2015) Quantification of neurotoxin BMAA (β-N-methylamino-L-alanine) in seafood from Swedish markets. Sci Rep 4:6931
Karamyan VT, Speth RC (2008) Animal models of BMAA neurotoxicity: A critical review. Life Sci 82:233–246
Karlsson O et al (2012) Neonatal exposure to the cyanobacterial toxin BMAA induces changes in protein expression and neurodegeneration in adult hippocampus. Toxicol Sci 130:391–404
Karlsson O, Lindquist NG, Brittebo EB, Roman E (2009a) Selective brain uptake and behavioral effects of the cyanobacterial toxin BMAA (β-N-Methylamino-L-alanine) following neonatal administration to rodents. Toxicol Sci 109:286–295
Karlsson O, Roman E, Brittebo EB (2009b) Long-Term Cognitive Impairments in Adult Rats Treated Neonatally with b -N-Methylamino- L -Alanine 112:185–195
Karlsson O, Jiang L, Andersson M, Ilag LL, Brittebo EB (2014) Protein association of the neurotoxin and non-protein amino acid BMAA (β-N-methylamino-l-alanine) in the liver and brain following neonatal administration in rats. Toxicol Lett 226:1–5
Khan FR, Alhewairini SS (1989) Zebrafish (Danio rerio) as a Model Organism. INTECH 32:137–144
Kim SY, Rydberg S (2020) Transfer of the neurotoxin β-N-methylamino-l-alanine (BMAA) in the agro-aqua cycle. Mar Drugs 18
Kolb A, Hildebrandt F, Lawrence C (2018) Effects of Diet and Social Housing on Reproductive Success in Adult Zebrafish. Danio Rerio Zebrafish 15:445–453
Langheinrich U (2003) Zebrafish: A new model on the pharmaceutical catwalk. BioEssays 25:904–912
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259:5301–5305
Martin RM, Stallrich J, Bereman MS (2019) Mixture designs to investigate adverse effects upon co-exposure to environmental cyanotoxins. Toxicology 421:74–83
Martin RM, Bereman MS, Marsden KC (2021) BMAA and MCLR Interact to Modulate Behavior and Exacerbate Molecular Changes Related to Neurodegeneration in Larval Zebrafish. Toxicol Sci 179:251–261
Masseret E et al (2013) Dietary BMAA exposure in an amyotrophic lateral sclerosis cluster from southern France. PLoS ONE 8:1–10
Mehta P et al (2022) Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Front Degener1–9
Metcalf JS et al (2017) Analysis of BMAA enantiomers in cycads, cyanobacteria, and mammals: in vivo formation and toxicity of d-BMAA. Amino Acids 49:1427–1439
Mondo K et al (2012) Cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) in Shark Fins. Mar Drugs 10:509–520
Mulder DW, Kurland LT, Offord KP, Beard M (1986) Familial adult motor neuron disease: Amyotrophic lateral sclerosis. Neurology 36:511–517
Murch SJ, Cox PA, Banack SA (2004a) A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. PNAS 101
Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW (2004b) Occurrence of b-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand 110:267–269
Neil JMO, Davis TW, Burford MA, Gobler CJ (2012) The rise of harmful cyanobacteria blooms : The potential roles of eutrophication and climate change. Harmful Algae 14:313–334
Nunn PB, Ponnusamy M (2009) β-N-Methylaminoalanine (BMAA): Metabolism and metabolic effects in model systems and in neural and other tissues of the rat in vitro. Toxicon 54:85–94
Neumann M et al. (2006) Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis
Okle O, Rath L, Galizia CG, Dietrich DR (2013) The cyanobacterial neurotoxin beta-N-methylamino-l-alanine (BMAA) induces neuronal and behavioral changes in honeybees. Toxicol Appl Pharmacol 270:9–15
Powers S, Kwok S, Lovejoy E, Lavin T, Sher RB (2017) Embryonic exposure to the environmental neurotoxin BMAA negatively impacts early neuronal development and progression of neurodegeneration in the Sod1-G93R zebrafish model of amyotrophic lateral sclerosis. Toxicol Sci 157:129–140
Price AL, Spencer CCA, Donnelly P (2015) Progress and promise in understanding the genetic basis of common diseases. Proc R Soc B Biol Sci 282
Ramesh T et al (2010) A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis Model Mech 3:652–662
Regueiro J, Negreira N, Carreira-casais A, Pérez-lamela C (2017) Dietary exposure and neurotoxicity of the environmental free and bound toxin β - N -methylamino- L -alanine. Food Res Int 100:1–13
Réveillon D et al (2015) β-N-methylamino-l-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon. French Mediterranean Sea Mar Environ Res 110:8–18
Rosen DR et al (1993) Mutations in Cu / Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nat 362:59–62
Roy U et al (2017) Metabolic profiling of zebrafish (Danio rerio) embryos by NMR spectroscopy reveals multifaceted toxicity of β-methylamino-L-alanine (BMAA). Sci Rep 7:17305
Santoriello C, Zon LI (2012) Science in medicine Hooked ! Modeling human disease in zebrafish. Sci Med 122:2337–2343
Scott LL, Downing TG (2017) A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases. Toxins (Basel) 10(22)
Sher RB (2017) The interaction of genetics and environmental toxicants in amyotrophic lateral sclerosis: Results from animal models. Neural Regen Res 12:902–905
Smith QR, Nagura H, Takada Y, Duncan MW (1992) Facilitated Transport of the Neurotoxin, P-N-Methylamino-L-Alanine. Across the Blood-Brain Barrier J Neurochem 58:1330–1337
Spencer PS et al (1987) Guam Amyotrophic Lateral Sclerosis--Parkinsonism--Dementia Linked to a Plant Excitant Neurotoxin. Science 237(80-. ):517–522
Tan VX et al (2018) Detection of the Cyanotoxins L-BMAA Uptake and Accumulation in Primary Neurons and Astrocytes. Neurotox Res 33
The ALS Association. https://www.als.org/understanding-als
van Onselen R, Downing TG (2018) BMAA-protein interactions: A possible new mechanism of toxicity. Toxicon 143:74–80
van Onselen R, Venables L, van de Venter M, Downing TG (2018) β-N-Methylamino-L-Alanine Toxicity in PC12: Excitotoxicity vs. Misincorporation Neurotox Res. https://doi.org/10.1007/s12640-017-9743-8
Vega A, Bell EA, Nunn PB (1968) The preparation of L-and D-alpha-amino-beta-methylaminopropionic acids and the identification of the compound isolated from cycas circinalis as the L-isomer. Phytochemistry 7:1885–1887
Wang S et al (2020) Toxicon Accumulation and distribution of neurotoxin BMAA in aquatic animals and effect on the behavior of zebrafish in a T-maze test. Toxicon 173:39–47
Wiltsie D, Schnetzer A, Green J, Vander Borgh M, Fensin E (2018) Algal blooms and cyanotoxins in Jordan Lake, North Carolina. Toxins (Basel) 10
Xie X, Basile M, Mash DC (2013) Cerebral uptake and protein incorporation of cyanobacterial toxin β-N-methylamino-L-alanine. NeuroReport 24:779–784
Acknowledgements
We are grateful to the late Dr. Christine Beattie of Ohio State University for the gift of the sodG93R transgenic zebrafish. Brain Chemistry Labs thanks the William C. and Joyce C. O’Neil Charitable Trust for support of this research. We thank members of the Planchart lab for helpful discussions during the execution of this work. We dedicate this work to the memory of Dr. Michael Bereman, who bravely fought ALS for 6 years and provided us with the inspiration for this work.
Funding
Ryan Weeks was supported by a Ruth L. Kirschstein National Research Service Award Institutional Training Grant (T32 ES007046); this work was supported in part by the Center for Human Health and the Environment (P30ES025128); funding for AP provided by N.C. State University. Paul Cox lab: William C. and Joyce C. O’Neil Charitable Trust.
Author information
Authors and Affiliations
Contributions
Ryan Weeks: primary researcher responsible for planning and conducting experiments, along with fish husbandry; assisted with mass spectrometry sample preparation. Sandra Banack: conducted all mass spectrometry experiments and analysis. Shaunacee Howell: aided in conducting behavioral experiments and fish husbandry. Preethi Thunga: responsible for swim tunnel data statistical analysis. Adrian Green: aided in experimental design and behavioral assays, responsible for light/dark assay data analysis. Paul Cox: collaborating PI who advised and directed mass spectrometry experiments. Antonio Planchart: primary PI responsible for project, including mentoring, experimental design, data analysis, and major funding.
Corresponding author
Ethics declarations
Ethical Approval
All fish husbandry, anesthesia, and euthanasia were conducted in accordance with NC State Institutional Animal Care and Use Committee (IACUC)-approved protocols.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weeks, R.D., Banack, S.A., Howell, S. et al. The Effects of Long-term, Low-dose β-N-methylamino-l-alanine (BMAA) Exposures in Adult SODG93R Transgenic Zebrafish. Neurotox Res 41, 481–495 (2023). https://doi.org/10.1007/s12640-023-00658-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-023-00658-z