Abstract
Introduction
Dexmedetomidine (DEX) is frequently used as an adjunct agent for prolonged sedation in the intensive care unit (ICU), though its effect on concomitant opioids or benzodiazepines infusions is unclear. We explored the impact of DEX on concomitant analgosedation in a cohort of ventilated pediatric patients in a cardiac ICU, with stratification of patients according to duration of ventilation (< 5 versus ≥ 5 days) following DEX initiation.
Methods
We conducted a retrospective analysis on ventilated patients receiving a DEX infusion ≥ 24 h and at least one other sedative/analgesic infusion (January 2011–June 2021). We evaluated trends of daily doses of opioids and benzodiazepines from 24 h before to 72 h following DEX initiation, stratifying patients based on ventilation duration after DEX initiation (< 5 versus ≥ 5 days).
Results
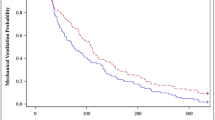
After excluding 1146 patients receiving DEX only, 1073 patients were included [median age 234 days (interquartile range 90, 879)]. DEX was associated with an opioid infusion in 99% of patients and a benzodiazepine infusion in 62%. Among patients ventilated for < 5 days (N = 761), opioids increased in the first 24 h following DEX initiation [+ 1.12 mg/kg/day (95% CI 0.96, 1.23), P < 0.001], then decreased [− 0.90 mg/kg/day (95% CI − 0.89, − 0.71), P < 0.001]; benzodiazepines slowly decreased [− 0.20 mg/kg/day (95% CI − 0.21, − 0.19), P < 0.001]. Among patients ventilated for ≥ 5 days (N = 312), opioid administration doubled [+ 2.09 mg/kg/day (95% CI 1.82, 2.36), P < 0.001] in the first 24 h, then diminished minimally [− 0.18 mg/kg/day (95% CI − 0.32, − 0.04), P = 0.015] without returning to baseline; benzodiazepine administration decreased minimally [− 0.03 mg/kg/day (95% CI − 0.05, − 0.01), P = 0.010]. Similar trends were confirmed when adjusting for age, gender, surgical complexity, recent major invasive procedures, duration of mechanical ventilation before DEX initiation, extubation within 72 h following DEX initiation, mean hourly DEX dose, and use of neuromuscular blocking infusion.
Conclusion
While in patients ventilated < 5 days opioids initially increased and then quickly decreased in the 72 h following DEX initiation, among patients ventilated ≥ 5 days opioids doubled, then decreased only minimally; benzodiazepines decreased minimally in both groups, although more slowly in the long-ventilation cohort. These findings may inform decision-making on timing of DEX initiation in ventilated patients already being treated with opioid or benzodiazepine infusions.


Similar content being viewed by others
References
Kudchadkar SR, Easley RB. Pain and sedation management. In: Nichols DG, Shaffner DH, editors. Rogers’ textbook of pediatric intensive care. 5th ed. London: Lippincott Williams & Wilkins; 2015.
Walker T, Kudchadkar SR. Pain and sedation management: 2018 Update for the Rogers’ textbook of pediatric intensive care. Pediatr Crit Care Med. 2019;20:54–61.
Mason KP, Lerman J. Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113:1129–42.
Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;2015:171–82. https://doi.org/10.1093/bja/aev226.
Buck ML, Willson D. Use of dexmedetomidine in the pediatric intensive care unit. Pharmacotherapy. 2008;28:51–7.
Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Pediatr Drugs. 2008;10:49–69.
Daverio M, et al. Pain and sedation management and monitoring in pediatric intensive care units across Europe: an ESPNIC survey. Crit Care. 2022;26:1–13.
Daverio M, Mondardini M, Sperotto F, Coscini N, Amigoni A. Use of dexmedetomidine for prolonged sedation in pediatric patients: a systematic review. Pediatr Crit Care Med. 2018;19:196.
Sperotto F, et al. Efficacy and safety of dexmedetomidine for prolonged sedation in the PICU. Pediatr Crit Care Med. 2020. https://doi.org/10.1097/pcc.0000000000002350.
Chrysostomou C, et al. Dexmedetomidine use in a pediatric cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med. 2009;10:654–60.
Horvath R, Halbrooks EF, Overman DM, Friedrichsdorf SJ. Efficacy and safety of postoperative dexmedetomidine administration in infants and children undergoing cardiac surgery: a retrospective cohort study. J Pediatr Infect Dis. 2015;1:138–45.
Gupta P, et al. Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease. Pediatr Crit Care Med. 2012;13:660–6.
Hosokawa K. Dexmedetomidine sedation in children after cardiac surgery*. Pediatric Crit Care Med. 2010;11:39–43.
Lam F, Bhutta AT, Tobias JD, Gossett JM, Morales L. Hemodynamic effects of dexmedetomidine in critically ill neonates and infants with heart disease. Pediatric Cardiol. 2012. https://doi.org/10.1007/s00246-012-0227-6.
Grant MJC, et al. Dexmedetomidine use in critically ill children with acute respiratory failure. Pediatr Crit Care Med. 2016;17:1131–41.
Sperotto F, Amigoni A. Dexmedetomidine for the treatment of delirium in the intensive care unit: do we need more evidence for adult and pediatric patients? Minerva Anestesiol. 2020;87:7–9.
Grant MJC, Balas MC, Curley MAQ. Defining sedation-related adverse events in the pediatric intensive care unit. Heart Lung. 2013;42:171–6.
Freriksen JJM, van der Zanden TM, Holsappel IGA, Molenbuur B, de Wildt SN. Best evidence-based dosing recommendations for dexmedetomidine for premedication and procedural sedation in pediatrics: outcome of a risk-benefit analysis by the Dutch pediatric formulary. Pediatr Drugs. 2022;24:247–57.
Smith HAB, et al. 2022 Society of Critical Care Medicine Clinical Practice Guidelines on Prevention and Management of Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Pediatric Patients with Consideration of the ICU Environment and Early Mobility. Pediatric Crit Care Med. 2022;23:e74–110.
Reade MC, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium a randomized clinical trial. JAMA. 2016;315:1460–8.
Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-dose nocturnal dexmedetomidine prevents ICU delirium a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018;197:1147–56.
Walker J, et al. Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res. 2004;27:206–10.
Shutes BL, Gee SW, Sargel CL, Fink KA, Tobias JD. Dexmedetomidine as single continuous sedative during noninvasive ventilation: typical usage, hemodynamic effects, and withdrawal. Pediatr Crit Care Med. 2018;19:287–97.
Sperotto F, et al. Prolonged sedation in critically ill children: is dexmedetomidine a safe option for younger age? An off-label experience. Minerva Anestesiol. 2019;85:164–72.
Curley MAQ, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379–89.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Reuth Nir, Francesca Sperotto, Manasee Godsay, Minmin Lu, and John N. Kheir, declare that they have no conflict of interest.
Ethics approval
The study was approved by the institutional review board of Boston Children’s Hospital (IRB P-00036098) under exemption from informed consent and was performed in accordance with the ethical standards included in the Declaration of Helsinki.
Funding
This study did not receive any internal nor external funding.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Data are available from the corresponding authors upon reasonable request.
Code availability
Not applicable.
Author contributions
Reuth Nir, MD collected the data, verified its accuracy, analyzed the data, wrote a first draft of the manuscript, and reviewed the final manuscript. Francesca Sperotto, MD, Ph.D. conceptualized the study, analyzed and interpreted the data, and wrote the final manuscript. Manasee Godsay, MS collected and verified the data, and reviewed the final manuscript. Minmin Lu, MS verified the accuracy and analyzed the data. John Kheir, MD conceptualized the study, supervised data collection, and wrote the final manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nir, R., Sperotto, F., Godsay, M. et al. Impact of Dexmedetomidine Infusion on Opioid and Benzodiazepine Doses in Ventilated Pediatric Patients in the Cardiac Intensive Care Unit. Pediatr Drugs 25, 709–718 (2023). https://doi.org/10.1007/s40272-023-00587-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00587-6