Abstract
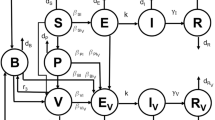
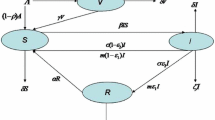
Compartmental models have long served as important tools in mathematical epidemiology, with their usefulness highlighted by the recent COVID-19 pandemic. However, most of the classical models fail to account for certain features of this disease and others like it, such as the ability of exposed individuals to recover without becoming infectious, or the possibility that asymptomatic individuals can indeed transmit the disease but at a lesser rate than the symptomatic. In the first part of this paper, we propose two new compartmental epidemiological models and study their equilibria, obtaining an endemic threshold theorem for the first model. In the second part of the paper, we treat the second model as an affine control system with two controls: vaccination and mitigation. We show that this system is static feedback linearizable, presents some simulations, and investigates an optimal control version of the problem. We conclude with some open problems and ideas for future research.







Similar content being viewed by others
Notes
All Mathematica and Maple code used in this paper is available upon request.
References
Agrachev A, Bonnard B, Chyba M, Kupka I (1997) Sub-Riemannian sphere in Martinet flat case. ESAIM Control Optim Calc Var 2:377–448
Anderson IM, Torre CG (2022) The differential geometry package. https://digitalcommons.usu.edu/dg/
Anderson RM, Vegvari C, Truscott J, Collyer BS (2021) Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 396:1614–1616
Aschwanden C (2021) Five reasons why COVID herd immunity is probably impossible. Nature 591:520–522
Avram F, Adenane R, Bianchin G, Halanay A (2022) Stability analysis of an eight parameter SIR-type model including loss of immunity, and disease and vaccination fatalities. Mathematics 10:402
Bjørnstad ON, Shea K, Krzywinski M, Altman N (2020) Modeling infectious epidemics. Nat Methods 17:455–456
Bjørnstad ON, Shea K, Krzywinski M, Altman N (2020) The SEIRS model for infectious disease dynamics. Nat Methods 17:557–559
Bonnard B (1991) Feedback equivalence for nonlinear systems and the time optimal control problem. SIAM J Control Optim 29:1300–1321
Bonnard B, Chyba M (2003) Singular trajectories and their role in control theory, vol 40. Mathematics and applications. Springer, Berlin, p 357
Britton T, Ball F, Trapman P (2020) A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science 369:846–849
Brockett RW (1978) Feedback invariants for nonlinear systems. IFAC Proc Vol 11:1115–1120
Bronisław J, Respondek W (1980) On linearization of control systems. L’Acad Pol Sci Bull Sér Sci Math 28:517–522
Brunovský P (1970) A classification of linear controllable systems. Kybernetika 6:173–188
Carney R, Chyba M, Fan V, Kunwar P, Lee T, Macadangdang I, Mileyko Y (2023) Modeling variants of the COVID-19 virus in Hawai’i and the responses to forecasting. AIMS Math 8:4487–4523
Caturano V et al (2021) Estimating asymptomatic SARS-CoV-2 infections in a geographic area of low disease incidence. BMC Infect Dis 21:1–4
Cheng Y, Yang X (2012) On the global stability of SEIRS models in epidemiology. Can Appl Math Q 20:115–133
Chengjun S, Ying-Hen H (2010) Global analysis of an SEIR model with varying population size and vaccination. Appl Math Model 34:2685–2697
Chyba M, Koniges A, Kunwar P, Lau W, Mileyko Y, Tong A (2022) COVID-19 heterogeneity in islands chain environment. PLoS ONE 17:e0263866
Elsner L, Monov V (2011) The bialternate matrix product revisited. Linear Algebra Appl 434:1058–1066
Fontanet A, Cauchemez S (2020) COVID-19 herd immunity: Where are we? Nat Rev Immunol 20:583–584
Fuller AT (2000) Conditions for a matrix to have only characteristic roots with negative real parts. SIAM Rev 42:599–653
Gandhi M, Yokoe DS, Havlir DV (2020) Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med 382:2158–2160
Gardner RB, Shadwick WF (1990) Feedback equivalence for general control systems. Syst Control Lett 15:15–23
Gardner RB, Shadwick WF (1991) An algorithm for feedback linearization. Differ Geom Appl 1:153–158
Gardner RB, Shadwick WF (1992) The GS algorithm for exact linearization to Brunovsky normal form. Inst Electr Electron Eng Trans Autom Control 37:224–230
Gardner RB, Shadwick WF, Wilkens GR (1983) Feedback equivalence and symmetries of Brunowski normal forms. Contemp Math 97:115–130
Gevertz JL, Greene JM, Sanchez Tapia CH, Sontag ED (2020) A novel COVID-19 epidemiological model with explicit susceptible and asymptomatic isolation compartments reveals unexpected consequences of timing social distancing. J Theor Biol 510:110539
Greene J, Sontag ED (2021) Minimizing the infected peak utilizing a single lockdown: a technical result regarding equal peaks. medRxiv. https://doi.org/10.1101/2021.06.26.21259589
Heffernan JM, Smith RJ, Wahl LM (2005) Perspectives on the basic reproductive ratio. J R Soc Interface 2:281–293
Hethcote HW (1968) The mathematics of infectious diseases. J Math Anal Appl 21:71–98
Hunt LR, Su R, Meyer G (1983) Design for multi-input nonlinear systems. Differ Geom Control Theory 27:268–298
Johansson MA et al (2021) SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 4:e2035057
Krener A (1973) On the equivalence of control systems and linearization of nonlinear systems. SIAM J Control Optim 11:670–676
Kunwar P, Markovichenko O, Chyba M, Mileyko Y, Koniges A, Lee T (2022) A study of computational and conceptual complexities of compartment and agent based models. Netw Heterog Media 17:359–384
Li MY, Muldowney JS (1995) Global stability for the SEIR model in epidemiology. Math Biosci 125:155–164
Li MY, Muldowney JS, van den Dreissche P (1999) Global stability of the SEIRS model in epidemiology. Can Appl Math Q 7:409–425
Liu WM, Hethcote HW, Levin SA (1987) Dynamical behavior of epidemiological models with nonlinear incidence rates. J Math Biol 25:359–380
Martcheva M (2015) An introduction to mathematical epidemiology. Springer, New York
Nogrady B (2020) What the data say about asymptomatic COVID infections. Nature 587:534–535
Pollock AM, Lancaster J (2020) Asymptomatic transmission of Covid-19. BMJ 371:m4851
Randolph HE, Barreiro LB (2020) Herd immunity: understanding COVID-19. Immunity 52:737–741
Sadeghi M, Greene JM, Sontag ED (2021) Universal features of epidemic models under social distancing guidelines. Annu Rev Control 51:426–440
Sontag ED (2021) An explicit formula for minimizing the infected peak in an SIR epidemic model when using a fixed number of complete lockdowns. Int J Robust Nonlinear Control Special Issue Control Theor Approaches Syst Life Sci 33:1–24
Vassiliou P (2006) A constructive generalised Goursat normal form. Differ Geom Appl 24:332–350
Vassiliou P (2006) Efficient construction of contact coordinates for partial prolongations. Found Comput Math 6:269–308
Acknowledgements
The first and third authors were supported by NSF Grant 2030789. The first three authors gratefully acknowledge support from the Coronavirus State Fiscal Recovery Funds via the Governor’s Office Hawaii Department of Defense.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Proof of Proposition 7
Appendix: Proof of Proposition 7
To find endemic equilibria of the SVE(R)IRS system, we need to solve the following system:
The third of the above equations readily yields \(I=(\sigma /\gamma )E\). Plugging this into the rest of the equations and setting \(\kappa = \frac{\sigma +\alpha \gamma }{n\gamma }\), we obtain
Note that \(E=0\) yields the disease-free equilibrium, so we may assume that \(E\ne 0\). Adding the bottom two equations to the first one and dividing the second equation by \(\beta \kappa E\), we get
From the first two equations, we easily find that
where
Using the expressions for S and V in terms of E, we obtain the following quadratic equation:
which yields the following two roots
Note that
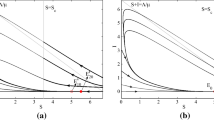
We thus see that if \(\mathfrak {R}_0>1\), then both roots are real, and \(E_{+}>0\), while \(E_{-}<0\). The latter gives a biologically irrelevant state. Computing the values of S and V from \(E_+\), we obtain
Rather than showing directly from these expressions that \(S_{+}\) and \(V_{+}\) are positive, we proceed as follows. Note that instead of expressing S and V in terms of E, we could as easily express any two of these variables in terms of the remaining one. Hence, we can obtain quadratic equations for S and V. We do not need the whole equations, but we note that both of them have a positive factor in front of the quadratic term, while the free constant term in the equation for V is \(\phi (\rho b -c)\), and in the equation for S, it is \(-(\rho b -c)(\beta \kappa c/a+\psi )/\rho \). Now, \(\rho b-c = \frac{\sigma +\delta }{\beta \kappa }>0\). Thus, the two roots of the equation for V have the same sign. Since \(E_{-}<0\) when \(\mathfrak {R}_0>1\), we see that one of such roots is \(b-a E_{-}>0\); hence, we also have \(V_{+}=b-a E_{+}>0\). Similarly, we note that if \(c\ge 0\), the two roots of the equation for S have opposite signs, and since one of them is \(-c+\rho a E_{-}<0\), we have \(S_{+}=-c+\rho a E_{+}>0\). The latter inequality is obvious when \(c<0\).
Finally, noting that \(I_{+}=(\sigma /\gamma )E_{+}>0\), we see that having \(\mathfrak {R}_0>1\) yields a single biologically relevant endemic equilibrium.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chyba, M., Klotz, T., Mileyko, Y. et al. A look at endemic equilibria of compartmental epidemiological models and model control via vaccination and mitigation. Math. Control Signals Syst. (2023). https://doi.org/10.1007/s00498-023-00365-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00498-023-00365-2