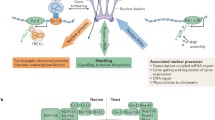
Abstract—Nucleocytoplasmic exchange in the cell occurs through the nuclear pore complexes (NPCs). NPCs are large multiprotein complexes with octagonal symmetry about their axis and imperfect mirror symmetry about a plane parallel with the nuclear envelop (NE). NPC fuses the inner and outer nuclear membranes and opens up a channel between nucleus and cytoplasm. NPC is built of nucleoporins. Each nucleoporin occurs in at least eight copies per NPC. Inside the NPC a permeability barrier forms by which NPCs can provide fast and selectable transport of molecules from one side of the nuclear membrane to the other. NPC architecture is based on hierarchical principle of organization. Nucleoporins are integrated into complexes that oligomerizes into bigger octomeric high-order structures. These structures are the main components of NPCs. In the first part of this work, the main attention is paid to NPC structure and nucleoporin properties. The second part is dedicated to mechanisms of NPC assembly and disassembly at different stages of the cell cycle.




Similar content being viewed by others
REFERENCES
Beck M., Hurt E. 2017. The nuclear pore complex: understanding its function through structural insight. Nat. Rev. Mol. Cell. Biol. 18 (2), 73‒89.
Hampoelz B., Andres-Pons A., Kastritis P., Beck M. 2019. Structure and assembly of the nuclear pore complex. Annu. Rev. Biophys. 48, 515‒536.
Allegretti M., Zimmerli C.E., Rantos V., Wilfling F., Ronchi P., Fung H.K.H., Lee C.W., Hagen W., Turoňová B., Karius K., Börmel M., Zhang X., Müller C.W., Schwab Y., Mahamid J., Pfander B., Kosinski J., Beck M. 2020. In-cell architecture of the nuclear pore and snapshots of its turnover. Nature. 586 (7831), 796‒800.
Schuller A.P., Wojtynek M., Mankus D., Tatli M., Kronenberg-Tenga R., Regmi S.G., Dip P.V., Lytton-Jean A.K.R., Brignole E.J., Dasso M., Weis K., Medalia O., Schwartz T.U. 2021. The cellular environment shapes the nuclear pore complex architecture. Nature. 598 (7882), 667‒671.
Wool I.G., Chan Y.L., Glück A. 1995. Structure and evolution of mammalian ribosomal proteins. Biochem. Cell. Biol. 73 (11‒12), 933‒947.
Callan H.G., Tomlin S.G. 1950. Experimental studies on amphibian oocyte nuclei. I. Investigation of the structure of the nuclear membrane by means of the electron microscope. Proc. R. Soc. London, Ser. B. 137 (888), 367‒378.
Dwyer N., Blobel G. 1976. A modified procedure for the isolation of a pore complex–lamina fraction from rat liver nuclei. J. Cell. Biol. 70 (3), 581‒591.
Akey C.W., Radermacher M. 1993. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J. Cell. Biol. 122 (1), 1‒19.
Rout M.P., Blobel G. 1993. Isolation of the yeast nuclear pore complex. J. Cell. Biol. 123 (4), 771‒783.
Yang Q., Rout M.P., Akey C.W. 1998. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol. Cell. 1 (2) 223‒234.
Cronshaw J.M., Krutchinsky A.N., Zhang W., Chait B.T., Matunis M.L.J. 2002. Proteomic analysis of the mammalian nuclear pore complex. J. Cell. Biol. 158 (5), 915‒927.
Rout M.P., Aitchison J.D., Suprapto A., Hjertaas K., Zhao Y., Chait B.T. 2000. The yeast nuclear pore complex: composition, architecture, transport mechanism. J. Cell. Biol. 148 (4), 635‒651.
Gomez-Cavazos J.S., Hetzer M.W. 2015. The nucleoporin gp210/Nup210 controls muscle differentiation by regulating nuclear envelope/ER homeostasis. J. Cell. Biol. 208 (6), 671‒681.
Pritchard C.E.J., Fornerod M., Kasper L.H., van Deursen J.M.A. 2000. RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains. J. Cell Biol. 145 (2), 237‒254.
Griffis E.R., Altan N., Lippincott-Schwartz J., Powers M.A. 2002. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol. Biol. Cell. 13 (4), 1292‒1297.
Kendirgi F., Barry D.M., Griffis E.R., Powers M.A., Wente S.R. 2003. An essential role for hGle1 nucleocytoplasmic shuttling in mRNA export. J. Cell. Biol. 160 (7), 1029‒1040.
Griffis E.R., Craige B., Dimaano C., Ullman K.S., Powers M.A. 2004. Distinct functions domains within nucleoporins Nup153 and Nup98 mediate transcription-dependent mobility. Mol. Biol. Cell. 15 (4), 1991‒2002.
Capelson M., Liang Y., Schulte R., Mair W., Wagner U., Hetzer M.W. 2010. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 140 (3), 372‒383.
Kalverda B., Pickersgill H., Shloma V.V., Fornerod M. 2010. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 140 (3), 360‒371.
Brown C.R., Kennedy C.J., Delmar V.A., Forbes D.J., Silver P.A. 2008. Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev. 22 (5), 627‒639.
Ribbeck K., Görlich D. 2002. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 21 (11), 2664‒2671.
von Appen A., Beck M. 2016. Structure determination of the nuclear pore complex with three-dimensional cryo electron microscopy. J. Mol. Biol. 428 (10), 2001‒2010.
Stuwe T., Correia A.R., Lin D.H., Paduch M., Lu V.T., Kossiakoff A.A., Hoelz A. 2015. Architecture of the nuclear pore complex coat. Science. 347 (6226), 1148‒1152.
Siniossoglou S., Lutzmann M., Santos-Rosa H., Leonard K., Mueller S., Aebi U., Hurt E. 2000. Structure and assembly of the Nup84p complex. J. Cell. Biol. 149 (1), 41‒54.
von Appen A., Kosinski J., Sparks L., Ori A., DiGuilio A.L., Vollmer B., Mackmull M.T., Banterle N., Parca L.,Kastritis P., Buczak K., Mosalaganti S., Hagen W., Andres-Pons A., Lemke E.A., Bork P., Antonin W., Glavy J.S., Bui K.H., Beck M. 2015. In situ structural analysis of the human nuclear pore complex. Nature. 526 (7571), 140‒143.
Bui K.H., von Appen A., DiGuilio A.L., Ori A., Sparks L., Mackmull M.T., Bock T., Hagen W., Andrés-Pons A., Glavy J.S., Beck M. 2013. Integrated structural analysis of the human nuclear pore complex scaffold. Cell. 155 (6), 1233‒1243.
Rajoo S., Vallotton P., Onischenko E., Weis K. 2018. Stoichiometry and compositional plasticity of the yeast nuclear pore complex revealed by quantitative fluorescence microscopy. Proc. Natl. Acad. Sci. U. S. A. 115 (17), 3969‒3977.
Rasala B.A., Orjalo A.V., Shen Z., Briggs S., Forbes D.J. 2006. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc. Natl. Acad. Sci. U. S. A. 103 (47), 17801‒17806.
Rout M.P., Field M.C. 2017. The evolution of organellar coat complexes and organization of the eukaryotic cell. Annu. Rev. Biochem. 86, 637‒657.
Beck M., Baumeister W. 2016. Cryo-electron tomography: can it reveal the molecular sociology of cells in atomic detail? Trends Cell. Biol. 26 (11), 825‒837.
Mosalaganti S., Kosinski J.,Albert S., Schaffer M., Strenkert D., Salomé P.A., Merchant S.S., Plitzko J.M., Baumeister W., Engel B.D., Beck M. 2018. In situ architecture of the algal nuclear pore complex. Nat. Commun. 9 (1), 2361.
Adams R.L., Mason A.C., Glass L., Aditi, Wente S.R. 2017. Nup42 and IP 6 coordinate Gle1 stimulation of Dbp5/DDX19B for mRNA export in yeast and human cells. Traffic. 18 (12), 776‒790.
Fornerod M., van Deursen J., van Baal S., Reynolds A., Davis D., Murti K.G., Fransen J., Grosveld G. 1997. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 16 (4), 807‒816.
Lin D.H., Correia A.R., Cai S.W., Huber F.M., Jette C.A., Hoelz A. 2018. Structural and functional analysis of mRNA export regulation by the nuclear pore complex. Nat. Commun. 9 (1), 2319.
Port S.A., Monecke T., Dickmanns A., Spillner C., Hofele R., Urlaub H., Ficner R., Kehlenbach R.H. 2015. Structural and functional characterization of CRM1-Nup214 interactions reveals multiple FG-binding sites involved in nuclear export. Cell. Rep. 13 (4), 690‒702.
Ritterhoff T., Das H., Hofhaus G., Schröder R.R., Flotho A., Melchior F. 2016. The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes. Nat. Commun. 7, 11482.
Hutten S., Flotho A., Melchior F., Kehlenbach R.H. 2008. The Nup358-RanGAP complex is required for efficient importin α/β-dependent nuclear import. Mol. Biol. Cell. 19 (5), 2300‒2310.
Mahadevan K., Zhang H., Akef A., Cui X.A., Gueroussov S., Cenik C., Roth F.P., Palazzo A.F. 2013. RanBP2/Nup358 potentiates the translation of a subset of mRNAs encoding secretory proteins. PLoS Biol. 11 (4), e1001545.
Dharan A., Talley S., Tripathi A., Mamede J.I., Majetschak M., Hope T.J., Campbell E.M. 2016. KIF5B and Nup358 cooperatively mediate the nuclear import of HIV-1 during infection. PLoS Pathog. 12 (6), e1005700.
Krull S., Thyberg J., Björkroth B., Rackwitz H.R., Cordes V.C. 2004. Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol. Biol. Cell. 15 (9), 4261‒4277.
Krull S., Dörries J., Boysen B., Reidenbach S., Magnius L., Norder H., Thyberg J., Cordes V.C. 2010. Protein Tpr is required for establishing nuclear pore-associated zones of heterochromatin exclusion. EMBO J. 29 (10), 1659‒1673.
Stewart M. 2007. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell. Biol. 8 (3), 195‒208.
Eisenhardt N., Redolfi J., Antonin W. 2014. Interaction of Nup53 with Ndc1 and Nup155 is required for nuclear pore complex assembly. J. Cell. Sci. 127 (4), 908‒921.
Mansfeld J., Güttinger S., Hawryluk-Gara L.A., Panté N., Mall M., Galy V., Haselmann U., Mühlhäusser P., Wozniak R.W., Mattaj I.W., Kutay U., Antonin W. 2006. the conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol. Cell. 22 (1), 93‒103.
Mitchell J.M., Mansfeld J., Capitanio J., Kutay U., Wozniak R.W. 2010. Pom121 links two essential subcomplexes of the nuclear pore complex core to the membrane. J. Cell. Biol. 191 (3), 505‒521.
Tompa P. 2012. Intrinsically disordered proteins: a 10-year recap. Trends Biochem. Sci. 37 (12), 509‒516.
Tompa P. 2011. Unstructural biology coming of age. Curr. Opin. Struct. Biol. 21 (3), 419‒425.
van Roey K., Uyar B., Weatheritt R.J., Dinkel H., Seiler M., Budd A., Gibson T.J., Davey N.E. 2014. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem. Rev. 114 (13), 6733‒6778.
Davey N.E., Roey K.V., Weatheritt R.J., Toedt G., Uyar B., Altenberg B., Budd A., Diella F., Dinkel H., Gibson T.J. 2012. Attributes of short linear motifs. Mol. Biosyst. 8 (1), 268‒281.
Davey N.E., Cyert M.S., Moses A.M. 2015. Short linear motifs—ex nihilo evolution of protein regulation. Cell Commun. Signal. 13, 43.
Lin D.H., Stuwe T., Schilbach S., Rundlet E.J., Perriches T., Mobbs G., Fan Y., Thierbach K., Huber F.M., Collins L.N., Davenport A.M., Jeon Y.E., Hoelz A. 2016. Architecture of the symmetric core of the nuclear pore. Science. 352 (6283), aaf1015.
Fischer J., Teimer R., Amlacher S., Kunze R., Hurt E. 2015. Linker Nups connect the nuclear pore complex inner ring with the outer ring and transport channel. Nat. Struct. Mol. Biol. 22 (10) 774‒781.
Hülsmann B.B., Labokha A.A., Görlich D. 2012. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell. 150 (4), 738‒751.
Doucet C.M., Talamas J.A., Hetzer M.W. 2010. Cell cycle-dependent differences in nuclear pore complex assembly in Metazoa. Cell. 141 (6), 1030‒1041.
Linder M., Köhler M., Boersema P., Weberruss M., Wandke C., Marino J., Ashiono C., Picotti P, Antonin W., Kutay U. 2017. Mitotic disassembly of nuclear pore complexes involves CDK1- and PLK1-mediated phosphorylation of key interconnecting nucleoporins. Dev. Cell. 43 (2), 141‒156.
Laurell E., Beck K., Krupina K., Theerthagiri G., Bodenmiller B., Horvath P., Aebersold R., Antonin W., Kutay U. 2011. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell. 144 (4), 539‒550.
Belgareh N., Rabut G., Baï S.W., van Overbeek M., Beaudouin J., Daigle N., Zatsepina O.V., Pasteau F., Labas V., Fromont-Racine M., Ellenberg J., Doye V. 2001. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J. Cell Biol. 154 (6), 1147‒1160.
Katsani K.R., Karess R.E., Dostatni N., Doye V. 2008. In vivo dynamics of Drosophila nuclear envelope components. Mol. Biol. Cell. 19 (9), 3652‒3666.
Loïodice I., Alves A., Rabut G., van Overbeek M., Ellenberg J., Sibarita J.B., Doye V. 2004. The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Mol. Biol. Cell. 15 (7), 3333‒3344.
Joseph J., Liu S.T., Jablonski S.A., Yen T.J., Dasso M. 2004. The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol-. 14 (7), 611‒617.
Hattersley N., Cheerambathur D., Moyle M., Stefanutti M., Richardson A., Lee K.Y., Dumont J., Oegema K., Desai A. 2016. A nucleoporin docks protein phosphatase 1 to direct meiotic chromosome segregation and nuclear assembly. Dev. Cell. 38 (5), 463‒477.
Anderson D.J., Hetzer M.W. 2007. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell. Biol. 9 (10), 1160‒1166.
Otsuka S., Steyer A.M., Schorb M., Hériché J.K., Hossain M.J., Sethi S., Kueblbeck M., Schwab Y., Beck M., Ellenberg J. 2018. Postmitotic nuclear pore assembly proceeds by radial dilation of small membrane openings. Nat. Struct. Mol. Biol. 25 (1), 21‒28.
Franz C., Walczak R., Yavuz S., Santarella R., Gentzel M., Askjaer P., Galy V., Hetzer M., Mattaj I.W., Antonin W. 2007. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 8 (2), 165‒172.
Rasala B.A., Ramos C., Harel A., Forbes D.J. 2008. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol. Biol. Cell. 19 (9), 3982‒3996.
Capelson M., Hetzer M.W. 2009. The role of nuclear pores in gene regulation, development and disease. EMBO Rep. 10 (7), 697‒705.
Galy V., Askjaer P., Franz C., López-Iglesias C., Mattaj I.W. 2006. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr. Biol. 16 (17), 1748‒1756.
Walther T.C., Askjaer P., Gentzel M., Habermann A., Griffiths G., Wilm M., Mattaj I.W., Hetzer M. 2003. RanGTP mediates nuclear pore complex assembly. Nature. 424 (6949), 689‒694.
Dultz E., Zanin E., Wurzenberger C., Braun M., Rabut G., Sironi L., Ellenberg J. 2008. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J. Cell. Biol. 180 (5), 857‒865.
Hawryluk-Gara L.A., Shibuya E.K., Wozniak R.W. 2005. Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Mol. Biol. Cell. 16 (5), 2382‒2394.
Otsuka S., Bui K.H., Schorb M., Julius Hossain M., Politi A.Z., Koch B., Eltsov M., Beck M., Ellenberg J. 2016. Nuclear pore assembly proceeds by an inside-out extrusion of the nuclear envelope. Elife. 5 (5), e19071.
de Magistris P., Tatarek-Nossol M., Dewor M., Antonin W. 2018. A self-inhibitory interaction within Nup155 and membrane binding are required for nuclear pore complex formation. J. Cell. Sci. 131 (1), jcs208538.
Haraguchi T., Koujin T., Hayakawa T., Kaneda T., Tsutsumi C., Imamoto N., Akazawa C., Sukegawa J., Yoneda Y., Hiraoka Y. 2000. Live fluorescence imaging reveals early recruitment of emerin, LBR, RanBP2, and Nup153 to reforming functional nuclear envelopes. J. Cell. Sci. 113 (5), 779‒794.
Dultz E., Huet S., Ellenberg J. 2009. Formation of the nuclear envelope permeability barrier studied by sequential photoswitching and flux analysis. Biophys. J. 97 (7), 1891‒1897.
Kapinos L.E., Huang B., Rencurel C., Lim R.Y.H. 2017. Karyopherins regulate nuclear pore complex barrier and transport function. J. Cell. Biol. 216 (11), 3609‒3624.
Lowe A.R., Tang J.H., Yassif J., Graf M., Huang W.Y.C., Groves J.T., Weis K., Liphardt J.T. 2015. Importin-β modulates the permeability of the nuclear pore complex in a Ran-dependent manner. Elife. 2015 (4), e04052.
Makio T., Stanton L.H., Lin C.C., Goldfarb D.S., Weis K., Wozniak R.W. 2009. The nucleoporins Nup170p and Nup157p are essential for nuclear pore complex assembly. J. Cell. Biol. 185 (3), 459‒437.
Webster B.M., Colombi P., Jäger J., Lusk C.P. 2014. Surveillance of nuclear pore complex assembly by ESCR-T-III/Vps4. Cell. 159 (2), 388‒401.
Wente S.R., Blobel G. 1993. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J. Cell. Biol. 123 (2), 275‒284.
Vollmer B., Lorenz M., Moreno-Andrés D., Bodenhöfer M., De Magistris P., Astrinidis S.A., Schooley A., Flötenmeyer M., Leptihn S., Antonin W. 2015. Nup153 recruits the Nup107-160 complex to the inner nuclear membrane for interphasic nuclear pore complex assembly. Dev. Cell. 33 (6), 717‒728.
Dawson T.R., Lazarus M.D., Hetzer M.W., Wente S.R. 2009. ER membrane-bending proteins are necessary for de novo nuclear pore formation. J. Cell. Biol. 184 (5), 659‒675.
Vollmer B., Schooley A., Sachdev R., Eisenhardt N., Schneider A.M., Sieverding C., Madlung J., Gerken U., Macek B., Antonin W. 2012. Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly. EMBO J. 3120. 4072‒4084.
Hampoelz B., Mackmull M.T., Machado P., Ronchi P., Bui K.H., Schieber N., Santarella-Mellwig R., Necakov A., Andrés-Pons A., Philippe J.M., Lecuit T., Schwab Y., Beck M. 2016. Pre-assembled nuclear pores insert into the nuclear envelope during early development. Cell. 166 (3) 664‒678.
Onischenko E.A., Gubanova N.V., Kiseleva E.V., Hallberg E. 2005. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in Drosophila embryos. Mol. Biol. Cell. 16 (11), 5152‒5162.
Stafstrom J.P., Staehelin L.A. 1984. Dynamics of the nuclear envelope and of nuclear pore complexes during mitosis in the Drosophila embryo. Eur. J. Cell Biol. 34 (1), 179‒189.
D′Angelo M.A., Anderson D.J., Richard E., Hetzer M.W. 2006. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 312 (5772), 440‒443.
Cordes V.C., Rackwitz H.R., Reidenbach S. 1997. Mediators of nuclear protein import target karyophilic proteins to pore complexes of cytoplasmic annulate lamellae. Exp. Cell Res. 237 (2), 419‒433.
Funding
The review was written with a grant from the Russian Science Foundation (project No. 22-14-00270).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Abbreviations: NPC (Nuclear Pore Complex), nuclear pore complex; FG repeat (Phe-Gly repeat), a nucleoporin fragment enriched with phenylalanine and glycine residues; SLiM (Short Linear Motif), short (up to ten amino acids) fragments of a protein molecule that play a key role in the formation of interactions with other proteins; CDK (Cyclin dependent kinase), cyclin-dependent kinase; PLK (Polo-like kinase), Sex-like kinase; PP1 (Protein phosphatase 1), protein phosphatase 1; ELYS (Embryonic Large molecule derived from Yolk Sac), component of the Y-complex; EPR, endoplasmic reticulum; AL (Anullate Lamellae), cistern-like EPR elements; ALPC (Anullate Lamellae Pore Complex), non-nuclear pore complex.
Rights and permissions
About this article
Cite this article
Orlova, A.V., Georgieva, S.G. & Kopytova, D.V. Assembly and Disassembly of the Nuclear Pore Complex: A View from the Structural Side. Mol Biol 57, 572–583 (2023). https://doi.org/10.1134/S0026893323040131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323040131