Abstract
Background
Stomach adenocarcinoma (STAD) is a leading contributor of cancer death and severely endangers human health worldwide. MicroRNA (miRNA) has been validated to involve in the pathogenesis of STAD. However, the potency of miR-365b-3p in STAD remains to be studied.
Objective
The goal of this work is to illuminate the function and mechanism of miR-365b-3p in STAD progression. The qPCR assay was conducted to identify the expression level of miR-365b-3p in STAD. The effects of miR-365b-3p on cell proliferation, apoptosis and angiogenesis were estimated with CCK-8, colony formation, western blot, tube formation and wound healing assays. Molecular mechanism was explored by RIP and luciferase reporter assays.
Results
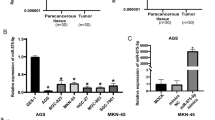
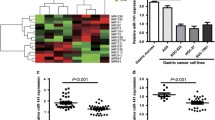
Low expression of miR-365b-3p in STAD was provoked by epigenetic modification. miR-365b-3p acted as a cancer suppressor in the development of STAD by inhibiting cell growth and angiogenesis. Functionally, STC1 was a direct effector for miR-365b-3p. More than that, miR-365b-3p was sponged by HCG18 and mediated the promoting role of HCG18 in STAD progression.
Conclusion
This study unraveled that miR-365b-3p exerted the inhibitory impacts on cell proliferation and angiogenesis of STAD through targeting STC1 and interacting with HCG18 for the first time, which broadened our understanding of STAD etiology and contributed to the treatment of STAD.









Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aggarwal T et al (2020) MicroRNAs as biomarker for breast cancer. Endocr Metab Immune Disord Drug Targets 20:1597–1610
Aguilar-Cazares D et al (2019) Contribution of angiogenesis to inflammation and cancer. Front Oncol 9:1399
Ajani JA et al (2017) Gastric adenocarcinoma. Nat Rev Dis Primers 3:17036
Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ (2020) Regulatory mechanism of microRNA expression in cancer. Int J Mol Sci 21:1723
Aprile G et al (2015) Angiogenic inhibitors in gastric cancers and gastroesophageal junction carcinomas: a critical insight. Crit Rev Oncol Hematol 95:165–178
Arigami T et al (2012) Expression of stanniocalcin 1 as a potential biomarker of gastric cancer. Oncology 83:158–164
Baek D et al (2008) The impact of microRNAs on protein output. Nature 455:64–71
Balatti V, Croce CM (2020) MicroRNA dysregulation and multi-targeted therapy for cancer treatment. Adv Biol Regul 75:100669
Bhan A, Soleimani M, Mandal SS (2017) Long noncoding RNA and cancer: a new paradigm. Cancer Res 77:3965–3981
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513: 202–9.
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Chang AC, Jellinek DA, Reddel RR (2003) Mammalian stanniocalcins and cancer. Endocr Relat Cancer 10:359–373
Chen X et al (2020) Use of a four-miRNA panel as a biomarker for the diagnosis of stomach adenocarcinoma. Dis Mark 2020:8880937
Chou MY et al (2015) Stanniocalcin-1 controls ion regulation functions of ion-transporting epithelium other than calcium balance. Int J Biol Sci 11:122–132
Ding B, Gao X, Li H, Liu L, Hao X (2017) A novel microRNA signature predicts survival in stomach adenocarcinoma. Oncotarget 8:28144–28153
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Fontana E, Sclafani F, Cunningham D (2014) Anti-angiogenic therapies for advanced esophago-gastric cancer. Indian J Med Paediatr Oncol 35:253–262
Fu T et al (2020) Identification of key long non-coding RNAs in gastric adenocarcinoma. Cancer Biomark 27:541–553
Guo W et al (2019) MiR-6872 host gene SEMA3B and its antisense lncRNA SEMA3B-AS1 function synergistically to suppress gastric cardia adenocarcinoma progression. Gastric Cancer 22:705–722
Han X, Liu H, Tang X, Zhao Y (2021) MiRNA-621 exerts tumor suppressor function in gastric adenocarcinoma by targeting AURKA/GSK-3β pathway. Acta Biochim Pol 68:91–98
He LF et al (2011) Stanniocalcin-1 promotes tumor angiogenesis through up-regulation of VEGF in gastric cancer cells. J Biomed Sci 18:39
He B et al (2020) miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci 16:2628–2647
Huang YK, Yu JC (2015) Circulating microRNAs and long non-coding RNAs in gastric cancer diagnosis: an update and review. World J Gastroenterol 21:9863–9886
Hussain S, Zahra Bokhari SE, Fan XX, Malik SI (2021) The role of exosomes derived miRNAs in cancer. J Pak Med Assoc 71:1856–1861
Ilson DH (2014) Angiogenesis in gastric cancer: hitting the target? Lancet 383:4–6
Jászai J, Schmidt MHH (2019) Trends and challenges in tumor anti-angiogenic therapies. Cells 8:1102
Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ, Kauppila JH (2019) Tumor budding and prognosis in gastric adenocarcinoma. Am J Surg Pathol 43:229–234
Kim YJ et al (2019) Comparison of microRNA expression in tears of normal subjects and Sjögren syndrome patients. Invest Ophthalmol vis Sci 60:4889–4895
Kipkeeva FM et al (2020) A group of miRNA as candidates for prognostic biomarkers of gastric cancer metastasis. Bull Exp Biol Med 169:77–80
Kulkarni B et al (2019) Exosomal miRNA in chemoresistance, immune evasion, metastasis and progression of cancer. Drug Discov Today 24:2058–2067
Law AY, Wong CK (2013) Stanniocalcin-1 and -2 promote angiogenic sprouting in HUVECs via VEGF/VEGFR2 and angiopoietin signaling pathways. Mol Cell Endocrinol 374:73–81
Li CY et al (2017) Identification and functional characterization of microRNAs reveal a potential role in gastric cancer progression. Clin Transl Oncol 19:162–172
Li L, Ma TT, Ma YH, Jiang YF (2019) LncRNA HCG18 contributes to nasopharyngeal carcinoma development by modulating miR-140/CCND1 and Hedgehog signaling pathway. Eur Rev Med Pharmacol Sci 23:10387–10399
Li W, Pan T, Jiang W, Zhao H (2020) HCG18/miR-34a-5p/HMMR axis accelerates the progression of lung adenocarcinoma. Biomed Pharmacother 129:110217
Liu W, Xu J, Zhang C (2019) Clinical usefulness of gastric adenocarcinoma predictive long intergenic noncoding RNA in human malignancies: a meta-analysis. Pathol Res Pract 215:152387
Liu Y et al (2020) Long noncoding RNA HCG18 up-regulates the expression of WIPF1 and YAP/TAZ by inhibiting miR-141-3p in gastric cancer. Cancer Med 9:6752–6765
Maconi G, Manes G, Porro GB (2008) Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol 14:1149–1155
Mazumder S, Datta S, Ray JG, Chaudhuri K, Chatterjee R (2019) Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol 58:137–145
Min QH et al (2018) Differential expression of urinary exosomal microRNAs in IgA nephropathy. J Clin Lab Anal 32:e22226
Niu W et al (2020) E2F1-induced upregulation of lncRNA HCG18 stimulates proliferation and migration in gastric cancer by binding to miR-197-3p. Eur Rev Med Pharmacol Sci 24:9949–9956
Qu Y, Zhang N (2018) miR-365b-3p inhibits the cell proliferation and migration of human coronary artery smooth muscle cells by directly targeting ADAMTS1 in coronary atherosclerosis. Exp Ther Med 16:4239–4245
Ramassone A, Pagotto S, Veronese A, Visone R (2018) Epigenetics and microRNAs in cancer. Int J Mol Sci 19:459
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Sallas ML et al (2021) Dysregulated expression of apoptosis-associated genes and microRNAs and their involvement in gastric carcinogenesis. J Gastrointest Cancer 52:625–633
Shen S, Zhang S, Liu P, Wang J, Du H (2020) Potential role of microRNAs in the treatment and diagnosis of cervical cancer. Cancer Genet 248–249:25–30
Sitarz R et al (2018) Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 10:239–248
Slack FJ, Chinnaiyan AM (2019) The role of non-coding RNAs in oncology. Cell 179:1033–1055
Thomson DW, Dinger ME (2016) Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17:272–283
Titov SE, Anishchenko VV (2020) Differential diagnostics of gastric cancer and precancerous changes of the gastric mucosa using analysis of expression of six microRNAS. RCLD 65:131–136
Torre LA et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Wadhwa R, Taketa T, Sudo K, Blum MA, Ajani JA (2013) Modern oncological approaches to gastric adenocarcinoma. Gastroenterol Clin N Am 42:359–369
Wang J, Wang X, Wu G, Hou D, Hu Q (2013) MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle progression and apoptosis of human retinoblastoma cells by targeting PAX6. FEBS Lett 587:1779–1786
Wang Z, Jensen MA, Zenklusen JC (2016) A practical guide to the cancer genome atlas (TCGA). Methods Mol Biol 1418:111–141
Wang LX et al (2019a) Integrative analysis of long noncoding RNA (lncRNA), microRNA (miRNA) and mRNA expression and construction of a competing endogenous RNA (ceRNA) network in metastatic melanoma. Med Sci Monit 25:2896–2907
Wang Y et al (2019b) Stanniocalcin-1 promotes cell proliferation, chemoresistance and metastasis in hypoxic gastric cancer cells via Bcl-2. Oncol Rep 41:1998–2008
Wang L et al (2019c) Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci 20:5758
Yuan C, Zhang Y, Tu W, Guo Y (2019) Integrated miRNA profiling and bioinformatics analyses reveal upregulated miRNAs in gastric cancer. Oncol Lett 18:1979–1988
Yuan Z et al (2021) Long non-coding RNA HLA complex group 18 promotes gastric cancer progression by targeting microRNA-370–3p expression. J Pharm Pharmacol 74:250–258
Zha Z, Zhang P (2021) Identification and construction of a long noncoding RNA prognostic risk model for stomach adenocarcinoma patients. Dis Mark 2021:8895723
Zhang X et al (2021) MicroRNA-365b-3p represses the proliferation and promotes the apoptosis of non-small cell lung cancer cells by targeting PPP5C. Oncol Lett 21:389
Zheng Y et al (2021) miR-1262 suppresses gastric cardia adenocarcinoma via targeting oncogene ULK1. J Cancer 12:1231–1239
Acknowledgements
None.
Funding
This work was supported by University Yong Teacher Training Program Foundation of Henan Province, 2019 (No. 2019GGJS291); Key Scientific Research Projects Plan of Higher Education Institutions in Henan Province, 2023 (No. 23B310008); Medical Education Research Project, 2020 (No. Wjlx2020341) and Soft Science Research Project of Henan Province (No. 162400410524).
Author information
Authors and Affiliations
Contributions
HL, JS and HX designed the study. RH and YC collected the data and performed the statistical analysis. HL and JS wrote the manuscript. HX revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author HL declares that he has no conflict of interest. JS declares that he has no conflict of interest. RH declares that he has no conflict of interest. YC declares that he has no conflict of interest. HX declares that he has no conflict of interest.
Ethical approval
All included participants signed informed consent. The current work was carried out under the approval of the Ethics Committee of Xd Group Hospital. All experimental methods conformed to the requirements of the Animal Research Committee of Xd Group Hospital.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13273_2023_386_MOESM1_ESM.tif
Supplementary Figure 1 STC1 mediated the tumor suppressor effects of miR-365b-3p. (A) The transfection efficiency of STC1 overexpression was tested by qPCR and western blot in SNU216 cells. (B-C) The impacts of miR-365b-3p/STC1 on SNU216 cells proliferation were (B) CCK-8 and (C) colony formation assays. (D) Western blot was conducted to analyze the expression of apoptosis-related proteins. (E-G) The role of miR-365b-3p/STC1 in STAD angiogenesis was evaluated with (E) tube formation, (F) CCK-8 and (G) wound healing assays. Scale bar=100 μm. Each assay was repeated three times. **P<0.01, ***P<0.001. (TIF 2894 KB)
13273_2023_386_MOESM2_ESM.tif
Supplementary Figure 2 HCG18 facilitated STAD progression through inhibiting miR-365b-3p. (A) qPCR was carried out to measure the transfection efficiency of HCG18 overexpression in SNU216 cells. (B-C) The role of HCG18/miR-365b-3p in the proliferative capability of SNU216 cells was detected with (B) CCK-8 and (C) colony formation assays. (D) The expression of apoptosis-related proteins examined by western blot. (E-G) The influences of HCG18/miR-365b-3p on STAD angiogenesis were determined by (E) tube formation, (F) CCK-8 and (G) wound healing assays. Scale bar=100 μm. Each assay was repeated three times. *P<0.05, **P<0.01, ***P<0.001. (TIF 2813 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Song, J., Hou, R. et al. miR-365b-3p adsorbed by HCG18 inhibits the proliferation and angiogenesis of stomach adenocarcinoma by downregulating STC1. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00386-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00386-7