Abstract
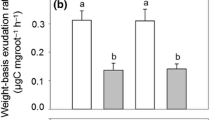
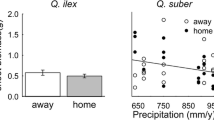
With global change already enhancing drought frequency and severity, there is a critical need to determine if temperate forests will continue to act as carbon (C) sinks. The degree to which belowground strategies sustain tree growth under water stress remains uncertain. We examined differences between two temperate forest tree species in their belowground response to experimentally reduced precipitation over three years. We chose trees that are dominant in temperate forests and have different belowground resource acquisition strategies. We tested our hypothesis that in response to reduced precipitation, sugar maple trees would reduce belowground C investment while oak trees would show resiliency belowground. We found that oak trees maintained belowground C investment at the same level, likely reflecting an ability to leverage a higher baseline level of investment to enhance water and nutrient uptake. By contrast, sugar maple trees initially responded with increased investment in roots and mycorrhizae, but mycorrhizal colonization faded over time. Moreover, the treatment reduced rhizodeposition belowground by sugar maple trees, suggesting that the belowground C investment we observed does not lead to increased mobilization of nutrients. Our findings indicate that there are species specific responses of C investment in roots, mycorrhizae, and rhizodeposits to reduced precipitation.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available in the EDI data repository. doi: https://doi.org/10.6073/pasta/4de59d4a3e7e4d81ed4398720f02202b.
References
Abrams MD (1992) Fire and the development of oak forests- in eastern North America, oak distribution reflects a variety of ecological paths and disturbance conditions. Bioscience 42:346–353
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012) The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc Natl Acad Sci USA 109:233–237
Anderegg WRL, Konings AG, Trugman AT, Yu K, Bowling DR, Gabbitas R, Karp DS, Pacala S, Sperry JS, Sulman BN, Zenes N (2018) Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 561:538–541
Au TF, Maxwell JT, Novick KA, Robeson SM, Warner SM, Lockwood BR, Phillips RP, Harley GL, Telewski FW, Therrell MD, Pederson N (2020) Demographic shifts in eastern US forests increase the impact of late-season drought on forest growth. Ecography 43:1475–1486
Averill C, Dietze MC, Bhatnagar JM (2018) Continental-scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Glob Change Biol 24(10):4544–4553
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644
Brzostek ER, Blair JM, Dukes JS, Frey SD, Hobbie SE, Melillo JM, Mitchell RJ, Pendall E, Reich PB, Shaver GR et al (2012) The effect of experimental warming and precipitation change on proteolytic enzyme activity: positive feedbacks to nitrogen availability are not universal. Glob Change Biol 18:2617–2625
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115:65–76
Brzostek ER, Dragoni D, Schmid HP, Rahman AF, Sims D, Wayson CA, Johnson DJ, Phillips RP (2014a) Chronic water stress reduces tree growth and the carbon sink of deciduous hardwood forests. Glob Change Biol 20(8):2531–2539
Brzostek ER, Fisher JB, Phillips RP (2014) Modeling the carbon cost of plant nitrogen acquisition: mycorrhizal trade-offs and multipath resistance uptake improve predictions of retranslocation. J Geophys Res: Biogeosci 119(8):1684–1697
Burton AJ, Pregitzer KS, Zogg GP, Zak DR (1998) Drought reduces root respiration in sugar maple forests. Ecol Appl 8(3):771–778
Carrara JE, Walter CA, Hawkins JS, Peterjohn WT, Averill C, Brzostek ER (2018) Interactions among plants, bacteria, and fungi reduce extracellular enzyme activities under long-term N fertilization. Glob Change Biol 24:2721–2734
Carrara JE, Fernandez IJ, Brzostek ER (2022) Mycorrhizal type determines root-microbial responses to nitrogen fertilization and recovery. Biogeochemistry 157:245–258
Casey NB (2020) Leaf angle and leaf stomata responses to experimental drought in Quercus velutina and Acer sccharum. West Virginia University, West Virginia
Cheeke TE, Phillips RP, Brzostek ER, Rosling A, Bever JD, Fransson P (2017) Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol 214(1):432–442
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG et al (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755
Clark JS, Iverson L, Woodall CW, Allen CD, Bell DM, Bragg DC, …, Zimmermann NE (2016) The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob Change Biol 22(7):2329–2352
Cleavitt NL, Battles JJ, Johnson CE, Fahey TJ (2018) Long-term decline of sugar maple following forest harvest, Hubbard Brook Experimental Forest, New Hampshire. Can J For Res 48(1):23–31
Comas LH, Callahan HS, Midford PE (2014) Patterns in root traits of woody species hosting arbuscular and ectomycorrhizas: implications for the evolution of belowground strategies. Ecol Evol 4:2979–2990
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Change 3(1):52–58. https://doi.org/10.1038/nclimate1633
Deng L, Peng C, Kim DG, Li J, Liu Y, Hai X, Liu Q, Huang C, Shangguan Z, Kuzyakov Y (2021) Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth Sci Rev 214:103501
Dickson RE, Tomlinson PT (1996) Oak growth, development and carbon metabolism in response to water stress. Ann des Sci For 53:181–196
Easterling DR, Arnold JR, Knutson T (2017) Precipitation change in the United States. Fourth Natl Clim Assess. https://doi.org/10.7930/J0H993CC
Fuchs S, Hertel D, Schuldt B, Leuschner C (2020) Effects of summer drought on the fine root system of five broadleaf tree species along a precipitation gradient. Forests 11:289
Gao D, Joseph J, Werner RA, Brunner I, Zurcher A, Hug C, Wang A, Zhao C, Bai E, Meusburger K, Gessler A, Hagedorn F (2021) Drought alters the carbon footprint of trees in soils- tracking the spatio-temporal fate of 13 C-labelled assimilates in the soil of an old-growth pine forest. Glob Change Biol 27(11):2491–2506
Gherardi LA, Sala OE (2020) Global patterns and climatic controls of belowground net carbon fixation. PNAS 117(13):20038–20043
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Hagedorn F, Joseph J, Peter M, Luster J, Pritsch K, Geppert U, Kerner R, Molinier V, Egli S, Schaub M et al (2016) Recovery of trees from drought depends on belowground sink control. Nat Plants 2:1–5
Hobbie EA (2006) Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology 87:563–569
Jo I, Fei S, Oswalt CM, Domke GM, Phillips RP (2019) Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci Adv 5(4):eaav6358
Kannenberg SA, Novick KA, Phillips RP (2018) Coarse roots prevent declines in whole-tree non-structural carbohydrate pools during drought in an isohydric and an anisohydric species. Tree Physiol 38(4):582–590
Keller AB, Phillips RP (2019) Relationship between belowground carbon allocation and nitrogen uptake in saplings varies by plant mycorrhizal type. Front For Glob Change 2:81
Keller AB, Brzostek ER, Craig ME, Fisher JB, Phillips RP (2021) Root-derived inputs are major contributors to soil carbon in temperate forests, but vary by mycorrhizal type. Ecol Lett 24:626–635
Knott JA, Desprez JM, Oswalt CM, Fei S (2019) Shifts in forest composition in the eastern United States. For Ecol Manag 433:176–183
Liese R, Leuschner C, Meier IC (2019) The effect of drought and season on root life span in temperate arbuscular mycorrhizal and ectomycorrhizal tree species. J Ecol 107:2226–2239
Liese R, Lübbe T, Albers NW, Meier IC (2018) The mycorrhizal type governs root exudation and nitrogen uptake of temperate tree species. Tree Physiol 38(1):83–95. https://doi.org/10.1093/treephys/tpx131
Matheny AM, Fiorella RP, Bohrer G, Poulsen CJ, Morin TH, Wunderlich A, Curtis PS (2017) Contrasting strategies of hydraulic control in two codominant temperate tree species. Ecohydrology 10(3):e1815
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular – arbuscular mycorrhizal Fungi. New Phytol 115:495–501
Meier IC, Leuschner C (2008) Belowground drought response of european beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14:2081–2095
Melillo JM, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L et al (2011) Soil warming, carbon-nitrogen interactions, and forest carbon budgets. Proc Natl Acad Sci USA 108:9508–9512
Nepstad DC (2002) The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J Phys Res 107:1–18
Phillips RP, Fahey TJ (2005) Patterns of rhizosphere carbon flux in sugar maple (Acer saccharum) and yellow birch (Betula allegheniensis) saplings. Glob Change Biol 11(6):983–995
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient coupling. New Phytol 199:41–51
Quinn Thomas R, Canham CD, Weathers KC, Goodale CL (2010) Increased tree carbon storage in response to nitrogen deposition in the US. Nat Geosci 3(1):13–17
Raczka NC, Carrara JE, Brzostek ER (2022) Plant-microbial responses to reduced precipitation depend on tree species in a temperate forest. Glob Change Biol 28(9):5820–5830
Roman DT, Novick KA, Brzostek ER, Dragoni D, Rahman F, Phillips RP (2015) The role of isohydric and anisohydric species in determining ecosystem-scale response to severe drought. Oecologia 179:641–654
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture (2020)
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12(1):46–53
Sokol NW, Sanderman J, Bradford MA (2019) Pathways of mineral-associated soil organic matter formation: integrating the role of plant carbon source, chemistry, and point of entry. Glob Change Biol 25(1):12–24
Stark JM, Firestone MK (1995) Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61:218–221
Sulman BN, Brzostek ER, Medici C, Shevliakova E, Menge DN, Phillips RP (2017) Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association. Ecol Lett 20(8):1043–1053
Terrer C, Vicca S, Stocker BD, Hungate BA, Phillips RP, Reich PB, Prentice IC (2018) Ecosystem responses to elevated CO2 governed by plant–soil interactions and the cost of nitrogen acquisition. New Phytol 217(2):507–522
Terrer C, Phillips RP, Hungate BA, Rosende J, Pett-Ridge J, Craig ME, Jackson RB (2021) A trade-off between plant and soil carbon storage under elevated CO2. Nature 591(7851):599–603
Thornthwaite CW (1948) An Approach toward a rational classification of climate. Geogr Rev 38:55
Treseder KK (2013) The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 371:1–13
Wang R, Cavagnaro TR, Jiang Y, Keitel C, Dijkstra FA (2021) Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J Ecol 109:3699–3709
Xu C, McDowell NG, Fisher RA, Wei L, Sevanto S, Christoffersen BO, Weng E, Middleton RS (2019) Increasing impacts of extreme droughts on vegetation productivity under climate change. Nat Clim Change 9:948–953
Yin H, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221
Yin L, Dijkstra FA, Phillips RP, Zhu B, Wang P, Cheng W (2021) Arbuscular mycorrhizal trees cause a higher carbon to nitrogen ratio of soil organic matter decomposition via rhizosphere priming than ectomycorrhizal trees. Soil Biol Biochem 157:108246
Acknowledgements
We acknowledge Rick Landenberger and the West Virginia Land Trust for logistical assistance and access to the experimental site in Tom’s Run Natural Area. We would like to acknowledge Kimberly Novick, Richard Phillips and Michael Chitwood for their assistance with throughfall exclusion shelter design. We thank Luis Andrés Guillen and Brittany Casey for their work in the field. We also thank Joanna Ridgeway, Emel Kangi, Hayden Starcher, Christopher Hughes, Dominick Cifelli, Stephen Oberle, Jordan Taylor, Muhammad Shammaa, Molly Chlovechok, Aaron Hull, Jessica Ries, Rachel McCoy, Sian Eisenhut, Hannah Minihan, and Kara Allen for assistance in the field and in the laboratory.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NCR and ERB developed core ideas and experimental design for this manuscript. NCR and JEC carried out field sample collection and experimental throughfall shelter upkeep. NCR completed sample analysis. NCR and CAW performed data analysis. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Caitlin Hicks Pries.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raczka, N.C., Walter, C.A., Carrara, J.E. et al. Divergent responses of belowground carbon investment in Quercus spp. and Acer saccharum to reduced precipitation. Biogeochemistry 165, 227–238 (2023). https://doi.org/10.1007/s10533-023-01078-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01078-z