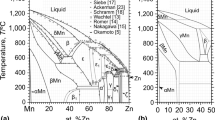
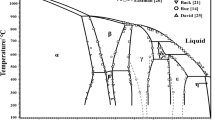
In the framework of the CALPHAD method, the thermodynamic assessment of the Cu–Ti–Hf system has been performed for the first time. This assessment considers the existence of homogeneity regions for Cu3Ti2, Cu4Ti3, CuTi, Cu5Hf, Cu51Hf14, and Cu10Hf7 compounds and the formation of a continuous solid solution of Cu(Ti, Hf)2 (γ-phase) in the ternary system. The thermodynamic assessments of the boundary binary systems and data on phase transformations and mixing enthalpy of melts in the ternary system became the basis for calculations. The Compound Energy Formalism was used to model the thermodynamic properties of intermetallic compounds with a homogeneity region. The associated solution model was used to describe the complex temperature dependence of the thermodynamic properties of melts from the temperature at which equilibrium melts exist to the glass-formation temperature. Upon the calculations, isothermal sections, vertical sections, projections of the liquidus and solidus surfaces, and reaction scheme of the phase diagram were presented. The liquid phase participates in eleven four-phase invariant reactions occurring in the temperature range 1138–1541 K. The diagrams of metastable phase transformations involving supercooled Cu–Ti–Hf melts and boundary solid solutions based on pure components were calculated. It is shown that supercooled melts in wide concentration ranges are thermodynamically stable in relation to boundary solid solutions based on pure components. The concentration region of glass formation for Cu–Ti–Hf melts by liquid quenching, predicted by the relative position of the \({T}_{0}^{L/\phi }\) and \({x}_{0}^{L/\phi }\) lines, is xCu ≈ 0.16–0.80.








Similar content being viewed by others
References
M. Sakata, N. Cowlam, and H.A. Davies, “Chemical short-range order in liquid and amorphous 66:34 copper–titanium alloys,” J. Phys. F: Met. Phys., 11, No. 7, L157–L162 (1981).
Ch.-H. Hwang, Y.-Jo Ryeom, and K. Cho, “Electrical resistivity and crystallization of amorphous Cu–Ti alloys,” J. Less-Common Met., 86, 187–194 (1982).
K.H.J. Buschow, “Effect of short-range ordering on the thermal stability of amorphous titanium–copper alloys,” Scr. Metall., 17, No. 9, 1135–1139 (1983).
J. Reeve, G.P. Gregan, and H.A. Davies, “Glass forming ability studies in the copper–titanium system,” in: Proc. 5th Int. Conf., Meeting Date 1984, North-Holland, Amsterdam (1985), Vol. 1, pp. 203–206.
C.G. Woychik, D.H. Lowndes, and T.B. Massalski, “Solidification structures in melt-spun and pulsed laser-quenched copper-titanium alloys,” Acta Metall., 33, No. 10, 1861–1871 (1985).
K. Aoki and T. Masumoto, Proc. Inst. Meet. Advanced Materials Research Society, Pittsburgh, PA (1989), Vol. 3, pp. 393–398.
C. Colinet, A. Pasturel, and K. Buschow, “Enthalpies of formation of Ti–Cu intermetallic and amorphous phases,” J. Alloys Compd., 247, No. 1, 15–19 (1997).
K.H.J. Buschow and N.M. Beekmans, “Thermal stability of amorphous alloys,” Solid State Commun., 35, No. 3, 233–236 (1980).
T. Zhang, A. Inoue, and T. Masumoto, “Amorphous (Ti, Zr, Hf) Ni Cu ternary alloys with a wide supercooled liquid region,” Mater. Sci. Eng. A, 181, 1423–1426 (1994).
A. Inoue, “High-strength Cu-based bulk glassy alloys in Cu–Zr–Ti and Cu–Hf–Ti ternary systems,” Acta Mater., 49, 2645–2652 (2001).
A. Inoue and W. Zhang, “Formation, thermal stability and mechanical properties of Cu–Zr and Cu–Hf binary glassy alloy rods,” Mater. Trans., 45, No. 2, 584–587 (2004).
R. Ristic, “Properties and atomic structure of amorphous early transition metals,” J. Alloys Compd., 504, S194–S197 (2010).
I.A. Figerua, H. Davies, and I. Todd, “Formation of Cu–Hf–Ti bulk metallic glasses,” J. Alloys Compd., 434, 164–166 (2007).
J. Basu and S. Ranganathan, “Glass forming ability and stability: Ternary Cu bearing Ti, Zr, Hf alloys,” Intermetallics, 17, No. 3, 128–135 (2009).
H. Choi-Yim and R. Conner, “Amorphous alloys in the Cu–Hf–Ti system,” J. Alloys Compd., 459, No. 1, 160–162 (2008).
M.A. Turchanin, P.G. Agraval, and A.R. Abdulov, “Thermodynamic assessment of the Cu–Ti–Zr system. I. Cu−Ti system,” Powder Metall. Met. Ceram., 47, No. 5–6, 344–360 (2008).
M.A. Turchanin and P.G. Agraval, “Thermodynamic assessment of the copper−hafnium system,” Powder Metall. Met. Ceram., 47, No. 3–4, 223–233 (2008).
H. Bittermann and P. Rogl, “Critical assessment and thermodynamic calculation of the ternary system boron–hafnium–titanium (B–Hf–Ti),” J. Phase Equilib., 18, No. 1, 24–47 (1997).
J.L. Liu, “Experimental investigation on phase equilibria of Cu–Ti–Hf system and performance of Cu (Ti, Hf) 2 phase,” J. Mater. Sci., 53, No. 10, 7809–7821 (2018).
V. Ronto, “Investigation of solidification behavior in Cu-based Cu–Hf–Ti alloy system,” IOP Conf. Ser.: Mater. Sci. Eng., 27, 012022 (2012).
V. Ronto, “Microstructure and phase analysis by TEM in Cu–Hf–Ti alloys,” Mater. Sci. Forum, 729, 266–271 (2013).
A.A. Vodopyanova, M.A. Turchanin, L.A. Dreval, and P.G. Agraval, “Partial and integral mixing enthalpies for liquid alloys in the Cu–Ti–Hf system,” Herald DSEA, No. 2 (41), 19–23 (2017).
A.T. Dinsdale, “SGTE data for pure elements,” CALPHAD, 15, No. 4, 317–425 (1991).
F. Sommer, “Homogeneous equilibria in liquid alloys and glass formation,” Ber. Bunsen Ges. Phys. Chem., 87, No. 9, 749–756 (1983).
M.A. Turchanin, I.V. Belokonenko, and P.G. Agraval, “On the application of the theory of ideal associated solution (TAS) for description of the temperature–concentration dependence of thermodynamic properties of binary melts,” Rasplavy, No. 1, 58–69 (2001).
A.A. Turchanin, M.A. Turchanin, and P.G. Agraval, “Thermodynamics of undercooled liquid and amorphous binary metallic alloys,” J. Metastable Nanocryst. Mater., 10, 481–486 (2001).
D.N. Saulov, I.G. Vladimirov, and A.Y. Klimenko, “Modified associate formalism without entropy paradox: Part I: Model description,” J. Alloys Compd., 473, No. 1–2, 167–175 (2009).
P.G. Agraval, L.A. Dreval, and M.A. Turchanin, “Interaction of components in Cu–Fe glass-forming melts with titanium, zirconium, and hafnium. II. Temperature–concentration dependence of thermodynamic mixing functions,” Powder Metall. Met. Ceram., 56, No. 5–6, 323–332 (2017).
M.A. Turchanin, I.V. Belokonenko, and P.G. Agraval, “Heats of formation of liquid alloys of nickel with IVA-metals,” Rasplavy, No. 3, 53–60 (2001).
M.A. Turchanin and P.G. Agraval, “Enthalpies of mixing of titanium, zirconium, and hafnium liquid alloys with cobalt,” Rasplavy, No. 2, 8–16 (2002).
M.A. Turchanin, P.G. Agraval, A.N. Fesenko, and A.R. Abdulov, “Thermodynamics of liquid alloys and metastable phase transformations in the copper–titanium system,” Powder Metall. Met. Ceram., 44, No. 5–6, 259–270 (2005).
M.A. Turchanin, “Phase equilibria and thermodynamics of binary copper systems with 3d-metals. VII. Concentration–temperature dependences of the thermodynamic functions of mixing for liquid alloys of copper and transition metals,” Powder Metall. Met. Ceram., 46, No. 11–12, 565–581 (2007).
M.A. Turchanin, P.G. Agraval, and A.R. Abdulov, “Thermodynamic assessment of the Cu–Ti–Zr system. II. Cu–Zr and Ti–Zr systems,” Powder Metall. Met. Ceram., 47, No. 7–8, 428–446 (2008).
M.A. Turchanin, “Temperature–composition dependence of thermodynamic mixing functions of liquid alloys of copper with rare-earth metals,” Powder Metall. Met. Ceram., 50, No. 7–8, 512–527 (2011).
P.G. Agraval, L.A. Dreval, and M.A. Turchanin, “Thermodynamic properties of iron melts with titanium, zirconium, and hafnium,” Powder Metall. Met. Ceram., 55, No. 11–12, 707–716 (2017).
M.A. Turchanin, T.Ya. Velikanova, P.G. Agraval, A.R. Abdulov, and L.A. Dreval’, “Thermodynamic assessment of the Cu–Ti–Zr system. III. Cu–Ti–Zr system,” Powder Metall. Met. Ceram., 47, No. 9–10, 586–606 (2008).
M. Turchanin, P. Agraval, L. Dreval, and A. Vodopyanova, “Calorimetric investigation of the mixing enthalpy of liquid Hf–Ni–Ti alloys and thermodynamic properties and chemical ordering in quaternary liquid Cu–Hf–Ni–Ti alloys,” J. Phase Equilib. Diffus., 41, No. 4, 469–490 (2020).
M. Turchanin, P. Agraval, L. Dreval, and A. Vodopyanova, “Thermodynamics and chemical ordering of liquid Cu–Hf–Ni–Ti–Zr alloys,” J. Phase Equilib. Diffus., 42, No. 5, 623–646 (2021).
M.A. Turchanin, L.O. Dreval, P.G. Agraval, V.A. Korsun, A.O. Vodopyanova, “Interaction of components in glass-forming melts of iron and nickel with titanium, zirconium, and hafnium. II. Temperature–concentration dependence of thermodynamic mixing functions of liquid alloys,” Powder Metall. Met. Ceram., 60, No. 11–12, 727–737 (2022).
L. Dreval, V. Korsun, P. Agraval, M. Turchanin, and A. Vodopyanova, “Thermodynamic mixing functions of the Fe–Ni–Zr liquid alloys,” Mater. Today: Proc., 62, No. P15, 7698–7702 (2022).
L. Dreval, V. Korsun, P. Agraval, A. Vodopyanova, and M. Turchanin, “Interaction of the components in liquid glass-forming Fe–Hf–Ni alloys,” J. Chem. Thermodyn., 173, No. 10, 106851 (2022).
M.A. Turchanin, P.G. Agraval, T.Ya. Velikanova, and A.A. Vodopyanova, “Predicting the composition ranges of amorphization for multicomponent melts in the framework of the CALPHAD method,” Powder Metall. Met. Ceram., 57, No. 1–2, 57–70 (2018).
Y.M. Muggianu, M. Gambino, and J.P. Bros, “Enthalpies of formation of liquid alloys bismuth–gallium–tin at 723 K. Choice of an analytical representation of integral and partial excess functions of mixing,” J. Chim. Phys. Phys.-Chim. Biol., 72, 83–88 (1975).
N. Saunders and A.P. Miodownik, CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide, Pergamon Press, Oxford (1998), p. 496.
M. Hillert, “The compound energy formalism,” J. Alloys Compd., 320, No. 2, 161–176 (2001).
R.B. Schwarz, P. Nash, and D. Turnbull, “The use of thermodynamic models in the prediction of the glass-forming range of binary alloys,” J. Mat. Res., 2, No. 04, 456–460 (1987).
R. Bormann, F. Gärtner, and K. Zöltzer, “Application of the CALPHAD method for the prediction of amorphous phase formation,” J. Less-Common Met., 145, 19–29 (1988).
N. Saunders and A.P. Miodownik, “Free energy criteria for glass forming alloys,” Ber. Bunsen Ges. Phys. Chem., 87, No. 9, 830–834 (1983).
P.G. Agraval, L.A. Dreval, and M.A. Turchanin, “Interaction of components in Cu–Fe glass-forming melts with titanium, zirconium, and hafnium. III. Modeling of metastable phase transformations with participation of liquid phase,” Powder Metall. Met. Ceram., 56, No. 7–8, 463–472 (2017).
G. Duan, D. Xu, and W.L. Johnson, “High copper content bulk glass formation in bimetallic Cu–Hf system,” Metall. Mater. Trans. A, 36, No. 2, 455–458 (2005).
K. Mondal and B. Murty, “On the parameters to assess the glass forming ability of liquids,” J. Non-Cryst. Solids, 351, No. 16–17, 1366–1371 (2005).
C. Chattopadhyay, “Critical evaluation of glass forming ability criteria,” Mater. Sci. Technol., 32, No. 4, 380–400 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 61, Nos. 11–12 (548), pp. 94–115, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turchanin, M.A., Agraval, P.G. & Vodopyanova, G.O. Thermodynamic Assessment of the Glass-Forming Cu–Ti–Hf System. Powder Metall Met Ceram 61, 708–726 (2023). https://doi.org/10.1007/s11106-023-00358-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-023-00358-5