Abstract
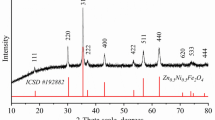
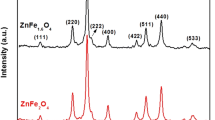
Nickel-zinc ferrites, which have pronounced ferrimagnetic and semiconductor properties, can be used as promising magnetically controlled photocatalysts for the purification of aqueous media from organic pollutants. The value of the specific surface area largely affects the photocatalytic properties of the material; therefore, the possibility of its control and variation at the stage of synthesis is of great scientific and technical interest. In this study, nanocrystalline ferrite of the Zn0.5Ni0.5Fe2O4 composition is obtained under conditions of solution combustion using various types of organic fuel as the main factor affecting the formation of the specific surface area, and subsequent heat treatment in air at a temperature of 500°C for 2 h. The crystal structure, chemical composition, and morphology of Zn0.5Ni0.5Fe2O4 are studied by methods of X‑ray phase analysis, X-ray spectral microanalysis, and scanning electron microscopy. The values of the specific surface area of the synthesized nanopowders are calculated based on the method of liquid-phase adsorption from a Methylene Blue solution and the low-temperature adsorption-desorption of nitrogen. The results of the X‑ray phase analysis show that a single-phase nanocrystalline product with a spinel structure is formed, where the average crystallite size varies within 11–23 nm and is inversely related to the value of the specific surface area, respectively, after the reaction with succinic acid (39.1 m2/g) and with glycine (20.2 m2/g). It is established that the choice of the fuel largely affects the formation of nanocrystals and the specific surface area of the samples, and the approach used makes it possible to control its values.






Similar content being viewed by others
REFERENCES
Martinson, K.D., Kozyritskaya, S.S., Panteleev, I.B., and Popkov, V.I., Low coercivity microwave ceramics based on LiZnMn ferrite synthesized via glycine–nitrate combustion, Nanosyst.: Phys. Chem. Math., 2019, vol. 10, no. 3, pp. 313–317.
Klumdoung, P., Hassadee, A., and Pankaew, P., Effect of nickel ferrite addition on characteristics of nanostructured nickel ferrite/hydroxyapatite ceramic, Mater. Today: Proc., 2019, vol. 17, pp. 1752–1760.
Hajarpour, S., Honarbakhsh, Raouf, A., and Gheisari, K., Structural evolution and magnetic properties of nanocrystalline magnesium–zinc soft ferrites synthesized by glycine-nitrate combustion and process, J. Magn. Magn. Mater., 2014, vol. 363, pp. 21–25.
Ombaka, L.M., Dillert, R., Robben, L., and Bahnemann, D.W., Evaluating carbon dots as electron mediators in photochemical and photocatalytic processes of NiFe2O4, APL Mater., 2020, vol. 8, no. 3, p. 031105.
Zulfiqar Ahmed, M.N., Chandrasekhar, K.B., Jahagirdar, A.A., Nagabhushana, H., and Nagabhushana, B.M., Photocatalytic activity of nanocrystalline ZnO, α‑Fe2O3 and ZnFe2O4/ZnO, Appl. Nanosci., 2015, vol. 5, no. 8, pp. 961–968.
Suppuraj, P., Parthiban, S., Swaminathan, M., and Muthuvel, I., Hydrothermal fabrication of ternary NrGO–TiO2/ZnFe2O4 nanocomposites for effective photocatalytic and fuel cell applications, Mater. Today: Proc., 2019, vol. 15, pp. 429–437.
Tsvetkov, M.P., Milanova, M.M., Cherkezova-Zheleva, Z.P., Tsoncheva, T.S., Zaharieva, J.T., Abrashev, M.V., and Mitov, I.G., Catalytic and photocatalytic properties of zinc–nickel and ferrites, J. Chem. Sci., 2021, vol. 133, p. 24.
Bohra, M., Alman, V., and Arras, R., Nanostructured ZnFe2O4: An exotic energy material, Nanomaterials, 2021, vol. 11, no. 5, p. 1286.
Ashiq, M.N., Naz, F., Malana, M.A., Gohar, R.S., and Ahmad, Z., Role of Co–Cr substitution on the structural, electrical and magnetic properties of nickel nano-ferrites synthesized by the chemical co-precipitation method, Mater. Res. Bull., 2012, vol. 47, no. 3, pp. 683–686.
Sunny, V., Kurian, P., Mohanan, P., Joy, P.A., and Anantharaman, M.R., A flexible microwave absorber based on nickel ferrite and nanocomposite, J. Alloys Compd., 2010, vol. 489, no. 1, pp. 297–303.
Ichiyanagi, Y., Uehashi, T., and Yamada, S., Magnetic properties of Ni–Zn ferrite nanoparticles, Phys. Status Solidi C, 2004, vol. 1, no. 12, pp. 3485–3488.
Wang, Z., Wang, J., Pan, Y., Liu, F., Lai, Y., Li, J., and Jiang, L., Preparation and characterization of a novel and recyclable InVO4/ZnFe2O4 composite for Methylene Blue removal by adsorption and visible-light photocatalytic degradation, Appl. Surf. Sci., 2020, vol. 501, p. 144006.
Casbeer, E., Sharma, V.K., and Li, X.Z., Synthesis and photocatalytic activity of ferrites under visible light: A review, Sep. Purif. Technol., 2012, vol. 87, pp. 1–14.
Choudhary, S., Bisht, A., and Mohapatra, S., Microwave-assisted synthesis of α-Fe2O3/ZnFe2O4/ZnO ternary hybrid nanostructures for photocatalytic applications, Ceram. Int., 2021, vol. 47, no. 3, pp. 3833–3841.
Mady, A.H., Baynosa, M.L., Tuma, D., and Shim, J.J., Facile microwave-assisted green synthesis of Ag–ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation, Appl. Catal., B, 2017, vol. 203, pp. 416–427.
Passi, M. and Pal, B., A review on CaTiO3 photocatalyst: Activity enhancement methods and photocatalytic applications, Powder Technol., 2021, vol. 388, pp. 274–304.
Falak, P., Hassanzadeh-Tabrizi, S.A., and Saffar-Teluri, A., Synthesis, characterization, and magnetic properties of ZnO–ZnFe2O4 nanoparticles with high photocatalytic and activity, J. Magn. Magn. Mater., 2017, vol. 441, pp. 98–104.
Kido, Y., Nakanishi, K., and Kanamori, K., Sol-gel synthesis of zinc ferrite-based xerogel monoliths with well-defined macropores, RSC Adv., 2013, vol. 3, no. 11, pp. 3661–3666.
Costa, A.C.F.M., Fagury-Neto, E., Morelli, M.R., and Kiminami, R.H.G.A., Microwave synthesis of Ni–Zn ferrite powders, Mater. Sci. Forum, 2003, vol. 416–418, no. 1, pp. 705–710.
Kumar, S., Singh, V., Aggarwal, S., Mandal, U.K., and Kotnala, R.K., Synthesis of nanocrystalline Ni0.5Zn0.5Fe2O4 ferrite and study of its magnetic behavior at different temperatures, Mater. Sci. Eng., B, 2010, vol. 166, no. 1, pp. 76–82.
Hwang, C.C., Tsai, J.S., and Huang, T.H., Combustion synthesis of Ni–Zn ferrite by using glycine and metal nitrates—investigations of precursor homogeneity, product reproducibility, and reaction mechanism, Mater. Chem. Phys., 2005, vol. 93, nos. 2–3, pp. 330–336.
Varma, A., Mukasyan, A.S., Rogachev, A.S., and Manukyan, K.V., Solution combustion synthesis of nanoscale materials, Chem. Rev., 2016, vol. 116, no. 23, pp. 14493–14586.
Tugova, E.A. and Karpov, O.N., Glycine-nitrate combustion engineering of neodymium cobaltite nanocrystals, Rare Met., 2021, vol. 40, no. 7, pp. 1778–1784.
Fan, G., Gu, Z., Yang, L., and Li, F., Nanocrystalline zinc ferrite photocatalysts formed using the colloid mill and hydrothermal technique, Chem. Eng. J., 2009, vol. 155, nos. 1–2, pp. 534–541.
Novikov, V.A. and Firsova, I.A., The influence of the restorator in the reaction of solvent synthesis by burning on the combustion parameters and the properties of the produced products, Sovrem. Mater., Tekh. Tekhnol., 2017, vol. 6, no. 14, p. 93–99.
Gavrilova, D.A., Gavrilova, M.A., Kondrashkova, I.S., and Panteleev, I.B., Influence of the composition, structure and morphology of ZnxMn1 – xFe2O4 nanocrystals on the magnetic properties, Ogneup. Tekh. Keram., 2021, nos. 11–12, pp. 18–24.
Akhtar, M.N., Saleem, M., and Khan, M.A., Al doped spinel and garnet nanostructured ferrites for microwave frequency C and X-band and applications, J. Phys. Chem. Solids, 2018, vol. 123, pp. 260–265.
Hegyesi, N., Vad, R.T., and Pukanszky, B., Determination of the specific surface area of layered silicates by Methylene Blue adsorption: The role of structure, pH and layer charge, Appl. Clay Sci., 2017, vol. 146, pp. 50–55.
Laboratornye raboty i zadachi po kolloidnoi khimii (Laboratory Works and Tasks in Colloid Chemistry), Frolov, Yu.G. and Grodskii, A.S., Eds., Moscow: Khimiya, 1986.
Amulya, M.A.S., Nagaswarupa, H.P., Kumar, M.R.A., Ravikumar, C.R., Prashantha, S.C., and Kusuma, K.B., Sonochemical synthesis of NiFe2O4 nanoparticles: Characterization and their photocatalytic and electrochemical applications, Appl. Surf. Sci. Adv., 2020, vol. 1, p. 100023.
Sonu Sharma, S., Dutta, V., Raizada, P., Hosseini-Bandegharaei, A., Thakur, V., Nguyen, V.-H., Vanle, Q., and Singh, P., An overview of heterojunctioned ZnFe2O4 photocatalyst for enhanced oxidative water and purification, J. Environ. Chem. Eng., 2021, vol. 9, no. 5, p. 105812.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gavrilova, M.A., Gavrilova, D.A., Kondrashkova, I.S. et al. Formation of Zn0.5Ni0.5Fe2O4 Nanocrystals in Conditions of Solution Combustion: Effect of the Type of Fuel on the Structure and Morphology. Glass Phys Chem 49, 394–401 (2023). https://doi.org/10.1134/S108765962360028X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S108765962360028X