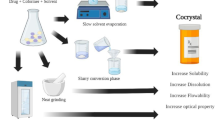
Abstract
Purpose
The primary objective of this research work was to formulate crystal engineered multicomponent form of a BCS II drug, telmisartan (TEL) using crystal engineering approach to improve its aqueous solubility. Further, it was attempted to understand the co-crystallization specificity of TEL using a set of structurally similar coformers.
Methods
Preliminary structural assessment and feasibility of cocrystal formation was done using Cambridge structural database (CSD) and molecular electrostatic surface potential (MESP). The formation of cocrystals was confirmed by different characterization techniques such as differential scanning calorimetry (DSC), hot stage microscopy (HSM), Fourier transform infrared spectroscopy (FTIR), and powder x-ray diffraction (PXRD). Solubility and dissolution studies were performed in phosphate buffer 7.5 and 0.1 N HCl.
Results
TEL cocrystal with maleic acid (MA) was successfully obtained, and co-crystallization specificity was decoded at MESP. Cocrystal structure was also solved from PXRD data. A 4.27-fold and 2.8-fold improvement in the solubility was observed in phosphate buffer 7.5 and 0.1 N HCl.
Conclusion
A significant improvement in the aqueous solubility and dissolution profile was observed for the prepared cocrystals over the pure TEL. The present study was a small initiative to serve as a guidance for the rationalized screening and preparation of novel multicomponent solids with desired properties.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
Abbreviations
- TEL:
-
Telmisartan
- DSC:
-
Differential scanning calorimetry
- HSM:
-
Hot stage microscopy
- FTIR:
-
Fourier transform infrared spectroscopy
- PXRD:
-
Powder x-ray diffraction
- API:
-
Active pharmaceutical ingredient
- MA:
-
Maleic acid
- FA:
-
Fumaric acid
- MAL:
-
Malonic acid
- MAC:
-
Malic acid
- SA:
-
Succinic acid
- MESP:
-
Molecular electrostatic surface potential
- DFT:
-
Density functional theory
- TASC:
-
Thermal analysis by structural characterization
References
Rohrer GS. Structure and bonding in crystalline materials. Cambridge University Press; 2001. https://doi.org/10.1017/CBO9780511816116.
Vippagunta SR, Brittain HG, Grant DJ. Crystalline solids. Adv Drug Deliv Rev. 2001;48(1):3–26. https://doi.org/10.1016/S0169-409X(01)00097-7.
Braga D, Grepioni F. Crystal engineering, Kirk‐Othmer Encyclopedia of Chemical Technology; 2000.
Desiraju GR. Crystal engineering: A holistic view. Angew Chem. 2007;46(44):8342–56. https://doi.org/10.1002/anie.200700534.
Desiraju GR, Vittal JJ, Ramanan A. Crystal engineering: A textbook. World Sci; 2011.
Desiraju GR. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew Chem, Int Ed Engl. 1995;34(21):2311–27. https://doi.org/10.1002/anie.199523111.
Nangia A, Desiraju GR. Supramolecular synthons and pattern recognition. Des Org Solids. 1998:57–95. https://doi.org/10.1007/3-540-69178-2_2.
Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9(6):2950–67. https://doi.org/10.1021/cg900129f.
Qiao N, Li M, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: An overview. Int J Pharm. 2011;419(1–2):1–1. https://doi.org/10.1016/j.ijpharm.2011.07.037.
Sokolov AN, Friščić T, MacGillivray LR. Enforced face-to-face stacking of organic semiconductor building blocks within hydrogen-bonded molecular cocrystals. J Am Chem Soc. 2006;128(9):2806–7. https://doi.org/10.1021/ja057939a.
Sekiya R, Kuroda R. Controlling stereoselectivity of solid-state photoreactions by co-crystal formation. Chem Comm. 2011;47(36):10097–9. https://doi.org/10.1039/C1CC13484A.
Millar DI, Maynard-Casely HE, Allan DR, Cumming AS, Lennie AR, Mackay AJ, Oswald ID, Tang CC, Pulham CR. Crystal engineering of energetic materials: Co-crystals of CL-20. CrystEngComm. 2012;14(10):3742–9. https://doi.org/10.1039/C2CE05796D.
Guo C, Zhang H, Wang X, Xu J, Liu Y, Liu X, Huang H, Sun J. Crystal structure and explosive performance of a new CL-20/caprolactam cocrystal. J Mol Struct. 2013;1048:267–73. https://doi.org/10.1016/j.molstruc.2013.05.025.
Nauha E, Ojala A, Nissinen M, Saxell H. Comparison of the polymorphs and solvates of two analogous fungicides—A case study of the applicability of a supramolecular synthon approach in crystal engineering. CrystEngComm. 2011;13(15):4956–64. https://doi.org/10.1039/C1CE05077J.
Braga D, Chelazzi L, Grepioni F, Nanna S, Rubini K, Curzi M, Giaffreda S, Saxell HE, Bratz M, Chiodo T. Imazamox: A quest for polymorphic modifications of a chiral and racemic herbicide. Cryst Growth Des. 2014;14(3):1430–7. https://doi.org/10.1021/cg4019025.
Good DJ, Rodriguez-Hornedo N. Solubility advantage of pharmaceutical cocrystals. Cryst Growth Des. 2009;9(5):2252–64. https://doi.org/10.1021/cg801039j.
Babu NJ, Nangia A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst Growth Des. 2011;11(7):2662–79. https://doi.org/10.1021/cg200492w.
Chadha R, Saini A, Arora P, Bhandari S. Pharmaceutical cocrystals: A novel approach for oral bioavailability enhancement of drugs. Crit Rev Ther Drug Carrier Syst. 2012;29(3). https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v29.i3.10
Müllers KC, Paisana M, Wahl MA. Simultaneous formation and micronization of pharmaceutical cocrystals by rapid expansion of supercritical solutions (RESS). Pharm Res. 2015;32:702–13. https://doi.org/10.1007/s11095-014-1498-9.
Bhatt JA, Bahl D, Morris K, Stevens LL, Haware RV. Structure-mechanics and improved tableting performance of the drug-drug cocrystal metformin: Salicylic acid. Eur J Pharm Biopharm. 2020;153:23–35. https://doi.org/10.1016/j.ejpb.2020.05.031.
Bolla G, Nangia A. Pharmaceutical cocrystals: Walking the talk. Chem Comm. 2016;52(54):8342–60. https://doi.org/10.1039/C6CC02943D.
Cherukuvada S, Babu NJ, Nangia A. Nitrofurantoin–p-aminobenzoic acid cocrystal: Hydration stability and dissolution rate studies. J Pharm Sci. 2011;100(8):3233–44. https://doi.org/10.1002/jps.22546.
Sanphui P, Devi VK, Clara D, Malviya N, Ganguly S, Desiraju GR. Cocrystals of hydrochlorothiazide: Solubility and diffusion/permeability enhancements through drug–coformer interactions. Mol Pharm. 2015;12(5):1615–22. https://doi.org/10.1021/acs.molpharmaceut.5b00020.
Bajaj A, Rao MR, Pardeshi A, Sali D. Nanocrystallization by evaporative antisolvent technique for solubility and bioavailability enhancement of telmisartan. AAPS PharmSciTech. 2012;13:1331–40. https://doi.org/10.1208/s12249-012-9860-x.
Lepek P, Sawicki W, Wlodarski K, Wojnarowska Z, Paluch M, Guzik L. Effect of amorphization method on telmisartan solubility and the tableting process. Eur J Pharm Biopharm. 2013;83(1):114–21. https://doi.org/10.1016/j.ejpb.2012.09.019.
Chadha R, Bhandari S, Haneef J, Khullar S, Mandal S. Cocrystals of telmisartan: Characterization, structure elucidation, in vivo and toxicity studies. CrystEngComm. 2014;16(36):8375–89. https://doi.org/10.1039/C4CE00797B.
Ganesh M, Ubaidulla U, Rathnam G, Jang HT. Chitosan-telmisartan polymeric cocrystals for improving oral absorption: In vitro and in vivo evaluation. Int J Biol Macromol. 2019;131:879–85. https://doi.org/10.1016/j.ijbiomac.2019.03.141.
Kundu S, Kumari N, Soni SR, Ranjan S, Kumar R, Sharon A, Ghosh A. Enhanced solubility of telmisartan phthalic acid cocrystals within the pH range of a systemic absorption site. ACS Omega. 2018;3(11):15380–8. https://doi.org/10.1021/acsomega.8b02144.
Haneef J, Arora P, Chadha R. Implication of coformer structural diversity on cocrystallization outcomes of telmisartan with improved biopharmaceutical performance. AAPS PharmSciTech. 2020;21:1–1. https://doi.org/10.1208/s12249-019-1559-9.
Haneef J, Chadha R. Drug-drug multicomponent solid forms: cocrystal, coamorphous and eutectic of three poorly soluble antihypertensive drugs using mechanochemical approach. AAPS PharmSciTech. 2017;18:2279–90. https://doi.org/10.1208/s12249-016-0701-1.
Musumeci D, Hunter CA, Prohens R, Scuderi S, McCabe JF. Virtual cocrystal screening. Chem Sci. 2011;2(5):883–90. https://doi.org/10.1039/C0SC00555J.
Dash SG, Thakur TS. Computational screening of multicomponent solid forms of 2-aryl-propionate class of NSAID, zaltoprofen, and their experimental validation. Cryst Growth Des. 2020;21(1):449–61. https://doi.org/10.1021/acs.cgd.0c01278.
Neese F. The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci. 2012;2(1):73–8. https://doi.org/10.1002/wcms.81.
Neese F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip Rev Comput Mol Sci. 2018;8(1):e1327. https://doi.org/10.1002/wcms.1327.
Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. 2012;33(5):580–92. https://doi.org/10.1002/jcc.22885.
Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14(1):33–8. https://doi.org/10.1016/0263-7855(96)00018-5.
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA. CrystalExplorer17.
Neumann MA. X-Cell: A novel indexing algorithm for routine tasks and difficult cases. J Appl Crystallogr. 2003;36(2):356–65.https://doi.org/10.1107/S0021889802023348.
Dinnebier RE, Sieger P, Nar H, Shankland K, David WI. Structural characterization of three crystalline modifications of telmisartan by single crystal and high-resolution X-ray powder diffraction. J Pharm Sci. 2000;89(11):1465–79. https://doi.org/10.1002/1520-6017.
Ramos JJ, Diogo HP. Thermal behavior and molecular mobility in the glassy state of three anti-hypertensive pharmaceutical ingredients. RSC Adv. 2017;7(18):10831–40. https://doi.org/10.1039/C7RA00298J.
Etter MC. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc Chem Res. 1990;23(4):120–6. https://doi.org/10.1021/ar00172a005.
Sherman AM, Geiger AC, Smith CJ, Taylor LS, Hinds J, Stroud PA, Simpson GJ. Stochastic differential scanning calorimetry by nonlinear optical microscopy. Anal Chem. 2019;92(1):1171–8.
Wang N, Huang X, Chen L, Yang J, Li X, Ma J, Bao Y, Li F, Yin Q, Hao H. Consistency and variability of cocrystals containing positional isomers: The self-assembly evolution mechanism of supramolecular synthons of cresol–piperazine. IUCrJ. 2019;6(6):1064–73. https://doi.org/10.1107/S2052252519012363.
Tomar D, Lodagekar A, Gunnam A, Allu S, Chavan RB, Tharkar M, Ajithkumar TG, Nangia AK, Shastri NR. The effects of cis and trans butenedioic acid on the physicochemical behavior of lumefantrine. CrystEngComm. 2022;24(1):156–68. https://doi.org/10.1039/D0CE01709D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Nanda, A. Similar but Not Same: Impact of Structurally Similar Coformers on Co-crystallization with Telmisartan. J Pharm Innov 18, 1954–1965 (2023). https://doi.org/10.1007/s12247-023-09759-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09759-w