Abstract
Purpose
The current investigative study was performed to analyze and compare the effect of gellan gum-mixed poloxamer 407 as compared to plain poloxamer 407 on the in situ gelling behavior as well as drug release of the best formulation.
Method
Azelastine hydrochloride-loaded poloxamer 407 micelles and poloxamer 407 gellan gum micelles were prepared with the help of thin film hydration followed by the characterization of the micelles by evaluating the particle size, entrapment efficiency, pH, and gelation as well as in vitro and in vivo drug release analysis.
Result
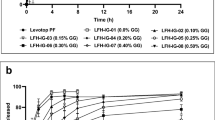
The azelastine hydrochloride poloxamer 407 with gellan gum micelles having a particle size below 100 nm were found to show low gelation temperature which further increases the retention time of the formulation at the site of administration and results in a controlled release formulation. The in vitro drug release showed significant release of 85% and 89% of drug in the 10th hour while the in vivo analysis showed similar effect as that of the marketed formulation of AzelastTM.
Conclusion
A controlled release in situ temperature sensitive gel was successfully formulated which showed significant effect to reduce allergic conjunctivitis.











Similar content being viewed by others
Data Availability
Data cannot be shared openly but are available on request from the corresponding author.
References
John JG, Devine DM, Kennedy KE, Geever LM, et al. The use of agar as a novel filler for monolithic matrices produced using hot melt extrusion. Eur J Pharm Biopharm. 2006;64(1):75–81. https://doi.org/10.1016/j.ejpb.2006.03.008.
Odeku OA. Assessment of Albizia zygia gum as a binding agent in tablet formulations. Acts Pharma. 2005;55(3):263–76.
Pawar HD, Mello PD. Isolation of seed gum from Cassia Tora and preliminary study of its application as a binder for tablets. Indian Drugs. 2004;41:465-468. https://www.researchgate.net/deref/https%3A%2F%2Fwww.researchgate.net%2F%25E2%2580%25A6%2F50338741_Introduction_to_the_Bioc
Odeku OA, Itiola OA. Evaluation of the effects of khaya gum on the mechanical and release properties of paracetamol tablets. Drug Dev Ind Pharm. 2003;29(3):311–20. https://doi.org/10.1081/ddc-120018205.
Kulkarni TG, Gowthamarajan K, Rao GB, Suresh B. Evaluation of binding properties of selected natural mucilages. Journal of Scientific and Industrial Research. 2022;61(7):529-532 Tripathi
Das N, Tripathi N, Basu S, Bose C et al. Progress in the development of gelling agents for improved culturability of microorganisms. Front. Microbiol. 2015;6: https://doi.org/10.3389/fmicb.2015.00698
Pathan IB, Chudiwal V, Farooqui I, Shingare P. Formulation design and evaluation of nasal in situ gel as a novel vehicle for Azelastine hydrochloride. International Journal of Drug Delivery. 2013;5(3):284–90.
Miyasaki S, Kawasaki N, Kubo W, Endo K, et al. Comparison of in situ gelling formulations for the oral delivery of cimetidine. Int J Pharm. 2001;220(1–2):161–8. https://doi.org/10.1016/s0378-5173(01)00669-x.
Agnihotri SA, Jawalkar SS, Aminabhavi ™. Controlled release of cephalexin through gellan gum beads: effect of formulation parameters on entrapment efficiency, size, and drug release. Eur J Pharm Biopharm. 2006;63(3):249-61. https://doi.org/10.1016/j.ejpb.2005.12.008
Dewan M, Sarkar G, Bhowmik M, Das B, et al. Effect of gellan gum on the thermogelation property and drug release profile of Poloxamer 407 based ophthalmic formulation. International Journal of Biological Macromolecules. 2017;102:258–65. https://doi.org/10.1016/j.ijbiomac.2017.03.194.
Milivojevic M, Pajic-Lijakovic I, Bugaraki B, Nayak AK et al. Chapter 6 - Gellan gum in drug delivery applications. Natural Polysaccharides in Drug Delivery and Biomedical Applications. 2019. Pp. 145-186. https://doi.org/10.1016/B978-0-12-817055-7.00006-6
Gupta H, Jain S, Mathur R, Mishra P, et al. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14(8):507–15. https://doi.org/10.1080/10717540701606426.
Cao Y, Zhang C, Shen W, Cheng Z, et al. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release. 2007;120(3):186–94. https://doi.org/10.1016/j.jconrel.2007.05.009.
Gupta S, Samanta MK, Raichur AM. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS PharmSciTech. 2010;11(1):322–35. https://doi.org/10.1208/s12249-010-9388-x.
Rokade M, Tambe B, Ruparel M. In situ gel-sustained nasal drug delivery. International Journal of Pharmaceutical Science and Research. 2015;4:4958–66.
Moghimipour E, Salimi A, Yousefvand T. Preparation and evaluation of celecoxib nanoemulsion for ocular drug delivery. Asian Journal of Pharmaceutics. 2017;11(3)(Suppl):S543
Khare P, Chogale MM, Kakade P, Patravale VB. Gellan gum–based in situ gelling ophthalmic nanosuspension of Posaconazole. Drug Deliv and Transl Res. 2022;12:2920–35. https://doi.org/10.1007/s13346-022-01155-0.
Gueudry J, Le Goff L, Lamoureux F, Pereira T, et al. Corneal pharmacokinetics of voriconazole and posaconazole following intrastromal injection and posaconazole eye drops instillation in rats. Current Eye Research. 2020;45(11):1369–72. https://doi.org/10.1080/02713683.2020.1749669.
Zhu L, Ao J, Li P. A novel in situ gel base of deacetylase gellan gum for sustained ophthalmic drug delivery of ketotifen: in vitro and in vivo evaluation. Drug Des Devel Ther. 2015;9:3943–3949. https://doi.org/10.2147/2FDDDT.S87368
Kidd M, McKenzie SH, Steven I, Cooper C, et al. Efficacy and safety of ketotifen eye drops in the treatment of seasonal allergic conjunctivitis. Br J Ophthalmol. 2003;87(10):1206–11. https://doi.org/10.1136/bjo.87.10.1206.
Barnes J, Moshirfar M. Timolol. [Updated 2022 Dec 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
Devi S, Saini V, Kumar M, Bhatt S, et al. A novel approach of drug localization through development of polymeric micellar system containing azelastine HCl for ocular delivery. Pharm Nanotechnology. 2019;20(4):231–45.
Williams PB, Crandall E, Sheppard JD. Azelastine hydrochloride, a dual-acting anti-inflammatory ophthalmic solution, for treatment of allergic conjunctivitis. Clin Ophthalmol. 2010;4:993–1001. https://doi.org/10.2147/opth.s13479.
Bodratti AM, Alexandridis P. Formulation of Poloxamers for Drug Delivery. J Funct Biomater. 2018;9(1):11. https://doi.org/10.3390/jfb9010011.
Patil S, Kadam A, Bandgar S, Patil S. Formulation and evaluation of an in situ gel for ocular drug delivery of anti conjunctival drug. Cellulose Chem Technol. 2015;49(1):35–40.
Dessi M, Borzacchiello A, Hashem T, Abdel-Fatah WI, et al. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. Journal of Biomedical Materials Research Part A. 2013;101(10):
Schwegmann H, Feitz AJ, Frimmel FH. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. Journal of Colloid and Interface Science. 2010;347(1):43-48. https://doi.org/10.1016/j.jcis.2010.02.028
Rasmussen MK, Pedersen JN, Marie R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat Commun. 2020;11:2337. https://doi.org/10.1038/s41467-020-15889-3.
Santosh SD, Manthan S, Nazeer N, Dharan SS. Formulation and assessment of ph triggered in situ ocular gel using selected fluoroquinolone antibiotic. J Pharm Sci & Res. 2020;12(10):1262–70.
Shinde UA, Shete JN, Nair HA, Singh KH. Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharm Dev Technol. 2014;19(7):813–23. https://doi.org/10.3109/10837450.2013.836217.
Singh RB, Liu L, Anchouche S, Yung A, et al. Ocular redness - I: Etiology, pathogenesis, and assessment of conjunctival hyperemia. Ocul Surf. 2021;21:134–44. https://doi.org/10.1016/j.jtos.2021.05.003.
Shoji J. Ocular allergy test and biomarkers on the ocular surface: clinical test for evaluating the ocular surface condition in allergic conjunctival diseases. Allergology International. 2020;69(4):496–504.
Elghobashy MR, Badran UM, Salem MY, Kelani KM. Stability indicating spectrophotometric and chromatographic methods for the determination of azelastine hydrochloride in presence of its alkaline degradant. Analytical Chemistry. 2014;14(4):135–42.
Cooper A. Heat capacity of hydrogen-bonded networks: an alternative view of protein folding thermodynamics. Biophys Chem. 2000;85(1):25–39. https://doi.org/10.1016/s0301-4622(00)00136-8.
Cooper A, Johnson CM, Lakey JH, Nöllmann M. Heat does not come in different colours: entropy-enthalpy compensation, free energy windows, quantum confinement, pressure perturbation calorimetry, solvation and the multiple causes of heat capacity effects in biomolecular interactions. Biophys Chem. 2001;93(2–3):215–30. https://doi.org/10.1016/s0301-4622(01)00222-8.
Cooper A. Biophysical Chemistry. London, UK: Royal Society of Chemistry. 2000;2004:103–7.
Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech. 2010;21(4):167–93.
Patil S, Ujalambkar V, Rathore A, Rojatkar S, et al. Galangin loaded galactosylated pluronic F68 polymeric micelles for liver targeting. Biomedicine & Pharmacotherapy. 2019;112:108691. https://doi.org/10.1016/j.biopha.2019.108691
Malekhosseini S, Rezaie A, Khaledian S, Abdoli M. Fabrication and characterization of hydrocortisone loaded Dextran-Poly Lactic-co-Glycolic acid micelle. Heliyon. 2020;6(5):e03975. http://dx.doi.org/https://doi.org/10.1016/j.heliyon.2020.e03975
Nagarwal RC, Singh SKPN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J Control Release. 2009;136(1):2–13. https://doi.org/10.1016/j.jconrel.2008.12.018.
Zhou H-Y,Hao J-L, Wang Y, Zhang W-S. Nanoparticles in the ocular drug delivery. Int J Opthalmol. 2013;6(3):390-396. https://doi.org/10.3980/2Fj.issn.2222-3959.2013.03.25
Wang K, Zhang T, Liu L, Wang X, et al. Novel micelle formulation of curcumin for enhancing antitumor activity and inhibiting colorectal cancer stem cells. International Journal of Nanomedicines. 2012;7:4487–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ranade, V., Dalal, Y., Palampalle, H.Y. et al. Formulation, Development, and Comparative Study of Azelastine-Loaded Temperature Sensitive In Situ Gelling Micelles for Allergic Conjunctivitis. J Pharm Innov 18, 1966–1980 (2023). https://doi.org/10.1007/s12247-023-09760-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09760-3