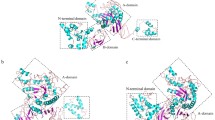
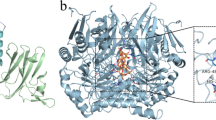
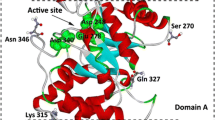
Abstract
Amaranthaceae α-amylase inhibitors (AAIs) are knottin-type proteins with selective inhibitory potential against coleopteran α-amylases. Their small size and remarkable stability make them exciting molecules for protein engineering to achieve superior selectivity and efficacy. In this report, we have designed a set of AAI pro- and mature peptides chimeras. Based on in silico analysis, stable AAI chimeras having a stronger affinity with target amylases were selected for characterization. In vitro studies validated that chimera of the propeptide from Chenopodium quinoa α-AI and mature peptide from Beta vulgaris α-AI possess 3, 7.6, and 4.26 fold higher inhibition potential than parental counterparts. Importantly, recombinant AAI chimera retained specificity towards target coleopteran α-amylases. In addition, to improve the inhibitory potential of AAI, we performed in silico site-saturation mutagenesis. Computational analysis followed by experimental data showed that substituting Asparagine at the 6th position with Methionine had a remarkable increase in the specific inhibition potential of Amaranthus hypochondriacus α-AI. These results provide structural–functional insights into the vitality of AAI propeptide and a potential hotspot for mutagenesis to enhance the AAI activity. Our investigation will be a toolkit for AAI’s optimization and functional differentiation for future biotechnological applications.






Similar content being viewed by others
References
Rane AS, Venkatesh V, Joshi RS, Giri AP (2020) International journal of biological macromolecules molecular investigation of coleopteran specific α-amylase inhibitors from amaranthaceae members. Int J Biol Macromol 163:1444–1450. https://doi.org/10.1016/j.ijbiomac.2020.07.219
Lu S, Deng P, Liu X et al (1999) Solution structure of the major α–amylase inhibitor of the crop plant amaranth *. J Biol Chem 274:20473–20478
Pereira PJB, Lozanov V, Patthy A et al (1999) Specific inhibition of insect α-amylases: yellow meal worm α-amylase in complex with the Amaranth α-amylase inhibitor at 2.0 Å resolution. Structure 7:1079–1088. https://doi.org/10.1016/S0969-2126(99)80175-0
Molesini B, Treggiari D, Dalbeni A et al (2017) Plant cystine-knot peptides: pharmacological perspectives. Br J Clin Pharmacol 83:63–70. https://doi.org/10.1111/bcp.12932
Rane AS, Joshi RS, Giri AP (2020) Molecular determinant for specificity: differential interaction of α-amylases with their Proteinaceous inhibitors. Biochim Biophys Acta (BBA)- General Subj. 1864:129703
Bhide AJ, Channale SM, Yadav Y et al (2017) Genomic and functional characterization of coleopteran insect-specific α-amylase inhibitor gene from Amaranthus species. Plant Mol Biol 94:319–332. https://doi.org/10.1007/s11103-017-0609-5
Svensson B, Fukuda K, Nielsen PK, Bønsager BC (2004) Proteinaceous α-amylase inhibitors. Biochim Biophys Acta—Proteins Proteomics 1696:145–156. https://doi.org/10.1016/j.bbapap.2003.07.004
Nguyen PQT, Ooi JSG, Nguyen NTK et al (2015) Antiviral cystine knot α-amylase inhibitors from Alstonia scholaris. J Biol Chem 290:31138–31150. https://doi.org/10.1074/jbc.M115.654855
Chagolla-Lopez A, Blanco-Labra A, Patthy A et al (1994) A novel α-amylase inhibitor from amaranth (Amaranthus hypocondriacus) seeds. J Biol Chem 269:23675–23680
Price-Carter M, Gray WR, Goldenberg DP (1996) Folding of ω-conotoxins. 1. efficient disulfide-coupled folding of mature sequences in vitro. Biochemistry 35:15537–15546. https://doi.org/10.1021/bi961574c
Price-Carter M, Gray WR, Goldenberg DP (1996) Folding of ω-conotoxins. 2. influence of precursor sequences and protein disulfide isomerase. Biochemistry 35:15547–15557. https://doi.org/10.1021/bi9615755
Buczek O, Olivera BM, Bulaj G (2004) Propeptide does not act as an intramolecular chaperone but facilitates protein disulfide isomerase-assisted folding of a conotoxin precursor †. Biochemistry 43:1093–1101
Berkut AA, Peigneur S, Myshkin MY et al (2015) Structure of membrane-active toxin from crab spider Heriaeus melloteei suggests parallel evolution of sodium channel gating modifiers in Araneomorphae and Mygalomorphae. J Biol Chem 290:492–504. https://doi.org/10.1074/jbc.M114.595678
Xu W, Meng Y, Surana P et al (2015) The knottin-like Blufensin family regulates genes involved in nuclear import and the secretory pathway in barley-powdery mildew interactions. Front Plant Sci 6:1–18. https://doi.org/10.3389/fpls.2015.00409
Bende NS, Dziemborowicz S, Herzig V et al (2015) The insecticidal spider toxin SFI1 is a knottin peptide that blocks the pore of insect voltage-gated sodium channels via a large β-hairpin loop. FEBS J282:904–920. https://doi.org/10.1111/febs.13189
Schwarz E (2017) Cystine knot growth factors and their functionally versatile proregions. Biol Chem 398:1295–1308
Clouse JW, Adhikary D, Page JT et al (2016) The amaranth genome: genome, transcriptome, and physical map assembly. Plant Genome 9:1. https://doi.org/10.3835/plantgenome2015.07.0062
Madeira F, Pearce M, Tivey ARN et al (2022) Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res 50:W276–W279. https://doi.org/10.1093/nar/gkac240
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324
Lobstein J, Emrich CA, Jeans C et al (2012) SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb Cell Fact 11:1. https://doi.org/10.1186/1475-2859-11-56
Channale SM, Bhide AJ, Yadav Y et al (2016) Characterization of two coleopteran α-amylases and molecular insights into their differential inhibition by synthetic α-amylase inhibitor, acarbose. Insect Biochem Mol Biol 74:1–11. https://doi.org/10.1016/j.ibmb.2016.04.009
Bhide AJ, Channale SM, Patil SS et al (2015) Biochimica et biophysica acta biochemical, structural and functional diversity between two digestive α -amylases from Helicoverpa armigera. BBA—Gen Subj 1850:1719–1728. https://doi.org/10.1016/j.bbagen.2015.04.008
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738. https://doi.org/10.1038/nprot.2010.5
Guex N, Peitsch MC, Schwede T (2009) Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30:162–173. https://doi.org/10.1002/elps.200900140
Pierce BG, Wiehe K, Hwang H et al (2014) ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 30:1771–1773. https://doi.org/10.1093/bioinformatics/btu097
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Pires DEV, Ascher DB, Blundell TL (2014) DUET: A server for predicting effects of mutations on protein stability using an integrated computational approach. Nucleic Acids Res 42:314–319. https://doi.org/10.1093/nar/gku411
Jo S, Kim T, Iyer VG, Im W (2008) CHARMMGUI A web-based graphical user interface for CHARMM. J Comput Chem 29:1859–1865
Joshi RS, Wagh TP, Sharma N et al (2014) Way toward “dietary pesticides”: molecular investigation of insecticidal action of caffeic acid against Helicoverpa armigera. J Agric Food Chem 62:10847–10854
Joshi RS, Mishra M, Tamhane VA et al (2014) The remarkable efficiency of a Pin-II proteinase inhibitor sans two conserved disulfide bonds is due to enhanced flexibility and hydrogen bond density in the reactive site loop. J Biomol Struct Dyn 32:13–26. https://doi.org/10.1080/07391102.2012.745378
Lansdowne LR, Beamer S, Jaczynski J, Matak KE (2009) Survival of Escherichia coli after isoelectric solubilization and precipitation of fish protein. J Food Prot 72:1398–1403. https://doi.org/10.4315/0362-028X-72.7.1398
Mi LZ, Brown CT, Gao Y et al (2015) Structure of bone morphogenetic protein 9 procomplex. Proc Natl Acad Sci USA 112:3710–3715. https://doi.org/10.1073/pnas.1501303112
Acknowledgements
ASR thanks the Department of Science and Technology under Women Scientist Scheme A (Project code:GAP320726). This project work is supported by the Rajiv Gandhi Science and Technology Commission(RGSTC/File-2018/DPP-184/CR-23), Mumbai, Government of Maharashtra, India. APG and RSJ acknowledge the Council of Scientific and Industrial Research (CSIR), India, and CSIR-National Chemical Laboratory (CSIR-NCL), Pune, India, for financial support under Project code MLP101526 and MLP036626.VSN and RSJ acknowledge the Science and Engineering Research Board, Department of Science and Technology, Government of India for financial support under Project code GAP336726. The authors acknowledge Nivedita Rai and Ananya Chouhan for their technical help.
Funding
Department of Science and Technology, India, GAP320726, Ashwini Rane, Council for Scientific and Industrial Research, India, MLP101526, Ashok P Giri, Council of Scientific and Industrial Research, India, MLP036626, Rakesh S. Joshi, Rajiv Gandhi Science and Technology Commission, RGSTC/File-2018/DPP-184/CR-23, Ashok P Giri, Science and Engineering Research Board, GAP336726, Rakesh S. Joshi
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest of any type.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rane, A.S., Nair, V.S., Joshi, R.S. et al. Domain Shuffling and Site-Saturation Mutagenesis for the Enhanced Inhibitory Potential of Amaranthaceae α-Amylase Inhibitors. Protein J 42, 519–532 (2023). https://doi.org/10.1007/s10930-023-10148-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-023-10148-y