Abstract
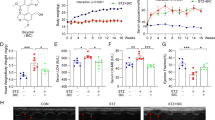
In diabetic patients, diabetic cardiomyopathy (DCM) is one of the most common causes of death. The inflammatory response is essential in the pathogenesis of DCM. Rhein, an anthraquinone compound, is extracted from the herb rhubarb, demonstrating various biological activities. However, it is unclear whether rhein has an anti-inflammatory effect in treating DCM. In our research, we investigated the anti-inflammatory properties as well as its possible mechanism. According to the findings in vitro, rhein could to exert an anti-inflammatory effect by reducing the production of NO, TNF-α, PGE2, iNOS, and COX-2 in RAW264.7 cells that had been stimulated with advanced glycosylation end products (AGEs). In addition, rhein alleviated H9C2 cells inflammation injury stimulated by AGEs/macrophage conditioned medium (CM). In vivo have depicted that continuous gavage of rhein could improve cardiac function and pathological changes. Moreover, it could inhibit the accumulation of AGEs and infiltration of inflammatory factors inside the heart of rats having DCM. Mechanism study showed rhein could suppress IKKβ and IκB phosphorylation via down-regulating TRAF6 expression to inhibit NF-κB pathway in AGEs/CM-induced H9C2 cells. Moreover, the anti-inflammation effect of rhein was realized through down-regulation phosphorylation of JNK MAPK. Furthermore, we found JNK MAPK could crosstalk with NF-κB pathway by regulating IκB phosphorylation without affecting IKKβ activity. And hence, the protective mechanism of rhein may involve the inhibiting of the TRAF6-NF/κB pathway, the JNK MAPK pathway, and the crosstalk between the two pathways. These results suggested that rhein may be a promising drug candidate in anti-inflammation and inflammation-related DCM therapy.
Graphical abstract










Similar content being viewed by others
Data availability statement
All data are available from the corresponding authors upon request.
Abbreviations
- AG:
-
Aminoguanidine
- AGEs:
-
Advanced glycation end products
- CM:
-
Macrophage conditioned medium
- COX-2:
-
Cyclooxygenase 2
- DCM:
-
Diabetic cardiomyopathy
- EF:
-
Ejection fraction
- EMPA:
-
Empagliflozin
- ERK:
-
Extra cellular regulated protein kinase
- FS:
-
Fractional shortening
- IKK:
-
IκB kinase
- iNOS:
-
Inducible nitric oxide synthase
- IκB:
-
Inhibitor of NF-κB
- LVIDd:
-
Left ventricular end-diastolic internal diameter
- LVIDs:
-
Left ventricular end-systolic internal diameters
- LVPWd:
-
Left ventricular posterior wall thickness during diastole
- LVPWs:
-
Left ventricular posterior wall thickness during systole
- JNK:
-
C-jun n-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MyD88:
-
Myeloid differentiation primary response gene 88
- MMP:
-
Mitochondrial membrane potential
- NF-κB:
-
Nuclear factor-κB
- PGE2 :
-
Prostaglandin E2
- STZ:
-
Streptozotocin
- T2DM:
-
Type 2 diabetes mellitus
- TLR4:
-
Toll-like receptors 4
- TNF-ɑ:
-
Tumor necrosis factor ɑ
- TRAF6:
-
The tumor necrosis factor receptor-associated factor 6
References
Kumar S, Mittal A, Babu D, Mittal A (2021) Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev 17(4):437–456. https://doi.org/10.2174/1573399816666201103143225
Sarraju A, Spencer-Bonilla G, Rodriguez F, Mahaffey KW (2021) Canagliflozin and cardiovascular outcomes in Type 2 diabetes. Future Cardiol 17(1):39–48. https://doi.org/10.2217/fca-2020-0029
Shah A, Isath A, Aronow WS (2022) Cardiovascular complications of diabetes. Expert Rev Endocrinol Metab 17(5):383–388. https://doi.org/10.1080/17446651.2022.2099838
Murtaza G, Virk H, Khalid M et al (2019) Diabetic cardiomyopathy—a comprehensive updated review. Prog Cardiovasc Dis 62(4):315–326. https://doi.org/10.1016/j.pcad.2019.03.003
Sun Y, Ding SZ (2021) NLRP3 inflammasome in diabetic cardiomyopathy and exercise intervention. Int J Mol Sci 22(24):13228. https://doi.org/10.3390/ijms222413228
Bodiga VL, Eda SR, Bodiga S (2014) Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart Fail Rev 19(1):49–63. https://doi.org/10.1007/s10741-013-9374-y
Wang Y, Luo W, Han J et al (2020) MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat Commun 11(1):2148. https://doi.org/10.1038/s41467-020-15978-3
Sun H, Luo GW, Chen DH, Xiang Z (2016) A comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front Pharmacol 7:247. https://doi.org/10.3389/fphar.2016.00247
Lai WW, Yang JS, Lai KC et al (2009) Rhein induced apoptosis through the endoplasmic reticulum stress, caspase- and mitochondria-dependent pathways in SCC-4 human tongue squamous cancer cells. In Vivo 23(2):309–316. https://doi.org/10.1089/hum.2008.138
Malaguti C, Vilella CA, Vieira KP et al (2008) Diacerhein downregulate proinflammatory cytokines expression and decrease the autoimmune diabetes frequency in nonobese diabetic (NOD) mice. Int Immunopharmacol 8(6):782–791. https://doi.org/10.1016/j.intimp.2008.01.020
Pei R, Jiang Y, Lei G et al (2021) Rhein derivatives, a promising pivot? Mini Rev Med Chem 21(5):554–575. https://doi.org/10.2174/1389557520666201109120855
Li Q, Su J, Jin SJ et al (2018) Argirein alleviates vascular endothelial insulin resistance through suppressing the activation of Nox4-dependent O2- production in diabetic rats. Free Radic Biol Med 121:169–179. https://doi.org/10.1016/j.freeradbiomed.2018.04.573
Wen Q, Miao J, Lau N et al (2020) Rhein attenuates lipopolysaccharide-primed inflammation through NF-κB inhibition in RAW264.7 cells: targeting the PPAR-γ signal pathway. Can J Physiol Pharmacol 98(6):357–365. https://doi.org/10.1139/cjpp-2019-0389
Lafuse WP, Wozniak DJ, Rajaram M (2020) Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells 10(1):51. https://doi.org/10.3390/cells10010051
Lentsch AB, Ward PA (1999) Activation and regulation of NFkappaB during acute inflammation. Clin Chem Lab Med 37(3):205–208. https://doi.org/10.1515/CCLM.1999.038
Singh S, Singh TG (2020) Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol 18(10):918–935. https://doi.org/10.2174/1570159X18666200207120949
Prantner D, Nallar S, Vogel SN (2020) The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways. FASEB J 34(12):15659–15674. https://doi.org/10.1096/fj.202002136R
Brown J, Wang H, Hajishengallis GN, Martin M (2011) TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res 90(4):417–427. https://doi.org/10.1177/0022034510381264
Nelson PT, Soma LA, Lavi E (2002) Microglia in diseases of the central nervous system. Ann Med 34(7–8):491–500. https://doi.org/10.1080/078538902321117698
Wilson AJ, Gill EK, Abudalo RA et al (2018) Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 104(4):293–299. https://doi.org/10.1136/heartjnl-2017-311448
Varga ZV, Giricz Z, Liaudet L et al (2015) Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852 2:232–242. https://doi.org/10.1016/j.bbadis.2014.06.030
Sumneang N, Apaijai N, Chattipakorn SC, Chattipakorn N (2021) Myeloid differentiation factor 2 in the heart: bench to bedside evidence for potential clinical benefits? Pharmacol Res 163:105239. https://doi.org/10.1016/j.phrs.2020.105239
Liu ZW, Wang JK, Qiu C et al (2015) Matrine pretreatment improves cardiac function in rats with diabetic cardiomyopathy via suppressing ROS/TLR-4 signaling pathway. Acta Pharmacol Sin 36(3):323–333. https://doi.org/10.1038/aps.2014.127
Youssef ME, Abdelrazek HM, Moustafa YM (2021) Cardioprotective role of GTS-21 by attenuating the TLR4/NF-κB pathway in streptozotocin-induced diabetic cardiomyopathy in rats. Naunyn Schmiedebergs Arch Pharmacol 394(1):11–31. https://doi.org/10.1007/s00210-020-01957-4
Feng B, Chen S, Gordon AD, Chakrabarti S (2017) miR-146a mediates inflammatory changes and fibrosis in the heart in diabetes. J Mol Cell Cardiol 105:70–76. https://doi.org/10.1016/j.yjmcc.2017.03.002
Shi H, Zhou P, Ni YQ, Wang SS et al (2021) In vivo and in vitro studies of Danzhi Jiangtang capsules against diabetic cardiomyopathy via TLR4/MyD88/NF-κB signaling pathway. Saudi Pharm J 29(12):1432–1440. https://doi.org/10.1016/j.jsps.2021.11.004
Kobayashi T, Walsh MC, Choi Y (2004) The role of TRAF6 in signal transduction and the immune response. Microbes Infect 6(14):1333–1338. https://doi.org/10.1016/j.micinf.2004.09.001
Chen X, Yu M, Xu W et al (2021) Rutin inhibited the advanced glycation end products-stimulated inflammatory response and extra-cellular matrix degeneration via targeting TRAF-6 and BCL-2 proteins in mouse model of osteoarthritis. Aging 13(18):22134–22147. https://doi.org/10.18632/aging.203470
Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25(6):280–288. https://doi.org/10.1016/j.it.2004.03.008
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132(3):344–362. https://doi.org/10.1016/j.cell.2008.01.020
Heintz L, Meyer-Schwesinger C (2021) The intertwining of autophagy and the ubiquitin proteasome system in podocyte (patho)physiology. Cell Physiol Biochem 55(S4):68–95. https://doi.org/10.33594/000000432
Wu Q, Feng Y, Ouyang Y et al (2021) Inhibition of advanced glycation endproducts formation by lotus seedpod oligomeric procyanidins through RAGE-MAPK signaling and NF-κB activation in high-AGEs-diet mice. Food Chem Toxicol 156:112481. https://doi.org/10.1016/j.fct.2021.112481
Zhu P, Ren M, Yang C et al (2012) Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol 21(2):123–129. https://doi.org/10.1111/j.1600-0625.2011.01408.x
Nonaka K, Kajiura Y, Bando M et al (2018) Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J Periodontal Res 53(3):334–344. https://doi.org/10.1111/jre.12518
Jeong YH, Kim Y, Song H et al (2014) Anti-inflammatory effects of α-galactosylceramide analogs in activated microglia: involvement of the p38 MAPK signaling pathway. PLoS ONE 9(2):e87030. https://doi.org/10.1371/journal.pone.0087030
Lee KM, Bang JH, Han JS et al (2013) Cardiotonic pill attenuates white matter and hippocampal damage via inhibiting microglial activation and downregulating ERK and p38 MAPK signaling in chronic cerebral hypoperfused rat. BMC Complement Altern Med 13:334. https://doi.org/10.1186/1472-6882-13-334
Rincón M, Davis RJ (2009) Regulation of the immune response by stress-activated protein kinases. Immunol Rev 228(1):212–224. https://doi.org/10.1111/j.1600-065X.2008.00744.x
Weston CR, Davis RJ (2002) The JNK signal transduction pathway. Curr Opin Genet Dev 12(1):14–21. https://doi.org/10.1016/s0959-437x(01)00258-1
Zhao H, Cheng L, Liu Y et al (2014) Mechanisms of anti-inflammatory property of conserved dopamine neurotrophic factor: inhibition of JNK signaling in lipopolysaccharide-induced microglia. J Mol Neurosci 52(2):186–192. https://doi.org/10.1007/s12031-013-0120-7
Yang Z, Lv J, Lu X et al (2018) Emulsified isoflurane induces release of cytochrome C in human neuroblastoma SHSY-5Y cells via JNK (c-Jun N-terminal kinases) signaling pathway. Neurotoxicol Teratol 65:19–25. https://doi.org/10.1016/j.ntt.2017.12.001
Zuo G, Ren X, Qian X et al (2019) Inhibition of JNK and p38 MAPK-mediated inflammation and apoptosis by ivabradine improves cardiac function in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol 234(2):1925–1936. https://doi.org/10.1002/jcp.27070
Ruan J, Qi Z, Shen L et al (2015) Crosstalk between JNK and NF-κB signaling pathways via HSP27 phosphorylation in HepG2 cells. Biochem Biophys Res Commun 456(1):122–128. https://doi.org/10.1016/j.bbrc.2014.11.045
Thompson EW (1988) Structural manifestations of diabetic cardiomyopathy in the rat and its reversal by insulin treatment. Am J Anat 182(3):270–282. https://doi.org/10.1002/aja.1001820308
Acknowledgements
The support of the experimental platform was generously provided by the Lingnan Medical Research Center of Guangzhou University of Chinese Medicine, and we are extremely thankful for this.
Funding
This study was funded by the National Natural Science Foundation of China for Young People (Nos. 82104621, 82204809) and Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515220066).
Author information
Authors and Affiliations
Contributions
S-ML and S-YZ conceived and designed the experiments. S-YZ carried out the experiments and wrote the manuscript. H-HZ participated in animal experiments. B-H provided the regents. CS and W-JP helped revise the article. All authors have read and agreed to the published version of the manuscript. S-YZ is the first author of this article. Correspondence should be addressed to S-YZ and S-ML.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics statement
The First Affiliated Hospital of Guangzhou University of Chinese Medicine's Experimental Animal Ethics Committee approved all of the experiments performed on animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11418_2023_1741_MOESM1_ESM.tif
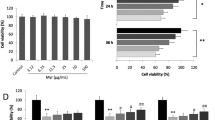
Supplementary file1 Fig. S1 RAW264.7 cells were treated with AGEs or rhein alone in a concentration or time-dependent manner. (A) RAW264.7 cells were treated with AGEs (from 0 to 350 μg/ml) for 8, 16, and 24 h respectively. NO production was measured. (B) RAW264.7 cells were treated with rhein (from 0 to 100 μM) for 24 h. cell viability was measured. N.S.: no significance. #P < 0.05, ###P < 0.001 compared to the control group (TIF 2847 KB)
11418_2023_1741_MOESM2_ESM.tif
Supplementary file2 Fig. S2 AGEs isn’t an ideal agent for inducing inflammatory injury in H9C2 cells. H9C2 cells were treated with AGEs (50–500 μg/ml) for 48 h, and the cell viability was then detected by MTT. Cell viabilities were similar between AGEs-treated H9C2 cells and DMEM-treated cells. (B–C) The releases of NO and LDH were detected by Griess and LDH Cytotoxicity Assay. ##P < 0.01 compared to the control group (TIF 4777 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, SY., Zhao, HH., Wang, BH. et al. Rhein alleviates advanced glycation end products (AGEs)-induced inflammatory injury of diabetic cardiomyopathy in vitro and in vivo models. J Nat Med 77, 898–915 (2023). https://doi.org/10.1007/s11418-023-01741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01741-7