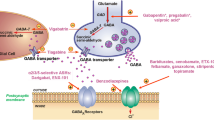
Abstract
The inhibitory neurotransmitter γ-aminobutyric acid (GABA) plays an important role in the modulation of neuronal excitability, and a disruption of GABAergic transmission contributes to the pathogenesis of some seizure disorders. Although many currently available antiseizure medications do act at least in part by potentiating GABAergic transmission, there is an opportunity for further research aimed at developing more innovative GABA-targeting therapies. The present article summarises available evidence on a number of such treatments in clinical development. These can be broadly divided into three groups. The first group consists of positive allosteric modulators of GABAA receptors and includes Staccato® alprazolam (an already marketed benzodiazepine being repurposed in epilepsy as a potential rescue inhalation treatment for prolonged and repetitive seizures), the α2/3/5 subtype-selective agents darigabat and ENX-101, and the orally active neurosteroids ETX155 and LPCN 2101. A second group comprises two drugs already marketed for non-neurological indications, which could be repurposed as treatments for seizure disorders. These include bumetanide, a diuretic agent that has undergone clinical trials in phenobarbital-resistant neonatal seizures and for which the rationale for further development in this indication is under debate, and ivermectin, an antiparasitic drug currently investigated in a randomised double-blind trial in focal epilepsy. The last group comprises a series of highly innovative therapies, namely GABAergic interneurons (NRTX-001) delivered via stereotactic cerebral implantation as a treatment for mesial temporal lobe epilepsy, an antisense oligonucleotide (STK-001) aimed at upregulating NaV1.1 currents and restoring the function of GABAergic interneurons, currently tested in a trial in patients with Dravet syndrome, and an adenoviral vector-based gene therapy (ETX-101) scheduled for investigation in Dravet syndrome. Another agent, a subcutaneously administered neuroactive peptide (NRP2945) that reportedly upregulates the expression of GABAA receptor α and β subunits is being investigated, with Lennox–Gastaut syndrome and other epilepsies as proposed indications. The diversity of the current pipeline underscores a strong interest in the GABA system as a target for new treatment development in epilepsy. To date, limited clinical data are available for these investigational treatments and further studies are required to assess their potential value in addressing unmet needs in epilepsy management.
Similar content being viewed by others
Despite the availability of more than 30 antiseizure medications, about one third of people with epilepsy do not achieve seizure freedom with existing pharmacological treatments. |
Potentiation of GABAergic inhibition using novel approaches is among the strategies being pursued in the effort to develop better treatments for seizures and epilepsy. |
Investigational treatments discussed in this article include novel therapeutic agents as well as drugs already in the market for non-epilepsy indications and being considered for repurposing in the treatment of seizure disorders. |
Several drugs in clinical development are novel positive allosteric modulators of GABAA receptors, aimed at taking advantage of the structural and functional heterogeneity of these receptors. These agents include novel neurosteroids and subunit-selective allosteric GABAA receptor modulators. |
Highly innovative treatments in early clinical development include an antisense oligonucleotide aimed at restoring the function of GABAergic interneurons in patients with Dravet syndrome, a cell therapy for mesial temporal lobe epilepsy based on stereotactically implanted GABAergic interneurons and a neuroprotective neuropeptide intended to upregulate GABA signalling. An adenoviral vector-based gene therapy is also scheduled for investigation in Dravet syndrome. |
Overall, the current pipeline of-γ-aminobutyric acid targeting agents includes many promising treatments, but for most of these, clinical data are too limited to infer their actual value in the therapeutic setting. |
1 Introduction
Because of the critical function of γ-aminobutyric acid (GABA) in modulating neuronal excitability [1] and the role played by a relative deficiency in GABA-mediated inhibition in the pathogenesis and expression of some seizure disorders [2,3,4], potentiation of GABAergic transmission has proved to be an attractive target for the development of new treatments for epilepsy for many years [5, 6]. Accordingly, the number of established antiseizure medications (ASMs) that act at least in part by enhancing GABAergic transmission has increased over time (Table 1) [5], with the two latest additions (cenobamate and ganaxolone) being discussed in detail in an accompanying article [6].
Despite the current availability of more than 30 ASMs, the pharmacological management of epilepsy continues to face major unmet needs. In particular, the second-generation ASMs introduced in the last 30 years have not reduced substantially the proportion of patients (about one third) who do not achieve seizure freedom with available medical treatments [7, 8]. Drug resistance continues to be highly prevalent in the most severe forms of epilepsy, particularly developmental and epileptic encephalopathies [8,9,10]. There is also a need for newer medications with improved tolerability and safety profiles, as well as for medications that could modify favourably the course of epilepsy rather than simply exerting a symptomatic effect against seizures [7]. During the last several decades, knowledge of the molecular biology of the GABA system has advanced greatly, in parallel with the understanding of the clinical implications of pharmacological manipulation of this system [4, 6]. Progress has also been made towards innovative approaches such as gene and cell therapies that could target the underlying aetiology of some epilepsies [11]. Overall, these advances have created attractive opportunities to address at least some of the unmet needs in epilepsy management. The purpose of the present article is to review investigational GABA-targeting therapies that are currently in clinical development for the treatment of seizure disorders. As it will be apparent from the pipeline discussion below, these include not only therapies that are entirely novel, but also drugs acting on the GABA system that are already in clinical use for other indications and are currently being investigated as potential repurposed treatments for seizures and epilepsy.
2 Novel Therapies in Clinical Development
2.1 Darigabat
Darigabat (CVL-865, PF-06372865) is an imidazopyridazine derivative, structurally unrelated to benzodiazepines and neurosteroids, which emerged from a search for novel GABAA-positive allosteric modulators retaining the anxiolytic and antiseizure properties of benzodiazepines while minimising undesirable effects such as somnolence, cognitive dysfunction, tolerance to therapeutic effects and abuse potential [12]. There is evidence that amnestic, sedative, ataxic and dependence-inducing effects of benzodiazepines are largely mediated by enhanced GABA signalling at GABAA receptors containing α1 subunits [13,14,15]. Conversely, anxiolytic, antiseizure and pain-modulating properties are mediated to an important extent by actions at α2 and α3 subunits [13,14,15,16,17,18], even though actions at the α1 subunit appear to also contribute to antiseizure effects, at least in some rodent models [13]. Based on these findings, efforts aimed at minimising undesirable properties of benzodiazepines have focused on the identification of positive allosteric modulators acting selectively at α2 subunits and α3 subunits [14]. In vitro, darigabat binds with high affinity at GABAA receptors containing α1, α2, α3 or α5 subunits, and has very low affinity for GABAA receptors containing α4 or α6 subunits, which do not have a benzodiazepine binding site [12]. However, functionally, darigabat exerts a low level of allosteric modulation at α1 subunits, and it behaves pharmacologically as an α2/3/5 subtype-selective, GABAA-positive allosteric modulator [12, 19, 20]. Its functional selectivity for α2/3/5 subunits relative to the α1 subunit has been confirmed in vivo by assessment of pharmacodynamic biomarkers in healthy subjects [19] and may contribute to an improved tolerability profile.
At doses of 0.3–10 mg/kg orally, darigabat showed dose-dependent anticonvulsant activity in the subcutaneous pentylenetetrazole test in mice [12, 21]. Darigabat (1 and 10 mg/kg orally) also showed antiseizure activity in the amygdala-kindled rat model of focal seizures [12, 21] and, at doses of 3 and 10 mg/kg orally, it showed comparable activity to diazepam (2 mg/kg intraperitoneally) in reducing hippocampal paroxismal discharges in the intra-hippocampal kainate mouse model of mesial temporal lobe epilepsy [22]. Darigabat (0.3–10 mg/kg orally) was also effective in reducing dose dependently spike‐and‐wave discharges in the Genetic Absence Rat from Strasbourg (GAERS) model of absence epilepsy, with complete suppression of epileptic discharges 30 min after administration of a 10-mg/kg dose [23], an effect that coupled with results obtained in other seizure models suggests broad-spectrum antiseizure activity.
In phase I dose-escalating studies in healthy subjects, darigabat (oral suspension) was absorbed rapidly following single doses of 0.04–100 mg, with peak plasma drug concentrations (Cmax) occurring at a median of 1–4 h after dosing [19]. The half-life of darigabat across these doses ranged from 6.0 to 8.9 h. Area under the plasma drug concentration–time curve and Cmax values appeared to increase dose proportionally within the dose range investigated. In these studies, mean oral clearance of darigabat ranged from 17.4 to 26.9 L/h, and its mean apparent volume of distribution ranged from 194 to 260 L [19]. Multiple-dose pharmacokinetics was assessed in a phase Ib study in 18 healthy subjects who received darigabat orally for 21 days [24]. Treatment included a 7-day titration phase followed by a 14-day maintenance phase at a dose of 25 mg twice daily (Cohort 1) or 42.5 mg twice daily (Cohort 2), with the higher dose expected to achieve a > 80% receptor occupancy [24]. Darigabat Cmax occurred at 1–2 h after dosing, with Cmax and area under the plasma drug concentration–time curve values during a dosing interval at steady state being approximately proportional to dose. The half-life of sarigabat assessed after stopping multiple-dose administration was about 11 h.
A summary of tolerability data from several studies in 136 healthy subjects and a total of 153 patients with a variety of conditions (photosensitive epilepsy, generalised anxiety disorder and chronic low back pain) enrolled in phase II trials concluded that darigabat is generally well tolerated. Dizziness and somnolence were the most commonly reported treatment-emergent adverse events (TEAEs), and could be minimised by including a short titration period upon initiation of therapy [20].
In a proof-of-concept, double-blind, randomised, placebo-controlled trial in seven patients with photosensitive epilepsy, single doses of darigabat (17.5 and 52 mg, expected to produce GABAA receptor of occupancy ≤ 60% and about 80%, respectively) administered as a tablet formulation produced a statistically significant reduction in the photoparoxismal electroencephalographic (EEG) response compared with placebo [25]. The response did not differ between the two doses, and was similar to that achieved with a single 2-mg oral dose of lorazepam. The effect was already present at the first assessment (1 hour after dosing) and persisted throughout the 6-hour assessment period. Six of the seven patients showed complete suppression of the photoparoxismal response with darigabat.
A phase II, randomised, placebo-controlled, double-blind adjunctive-therapy trial (NCT04244175) to evaluate the safety and efficacy of darigabat doses of 7.5 mg and 25 mg twice daily in 150 patients with drug-resistant focal seizures is ongoing. Darigabat is also being developed as a potential treatment for panic disorder [26]. With respect to other potential indicatients, darigabat failed to meet the primary efficacy endpoint in phase II placebo-controlled exploratory trials in patients with generalised anxiety disorder [27] and chronic low back pain [28].
2.2 ENX-101
ENX-101 is a subtype-selective GABAA receptor-positive allosteric modulator acting at receptors containing α2, α3 or α5 subunits, but devoid of positive allosteric activity at α1 subunit-containing receptors [29, 30]. The compound has been designed with the aim of maximising antiseizure activity while minimising the potential for sedation and tolerance, which are considered to be mediated largely by α1 subunit activation [31,32,33]. In a placebo-controlled phase I study in healthy volunteers, ENX-101 given orally once daily in the morning for 10 days at doses that ranged from 5 to 50 mg/day without titration was well tolerated, with evidence of persistent target engagement based on changes in a number of pharmacodynamic biomarkers [29]. The most commonly reported TEAE was mild and transient somnolence. The half-life of ENX-101 was estimated to be about 20 h. A phase II, randomised, placebo-controlled, double-blind, adjunctive-therapy trial to assess the efficacy and tolerability of ENX (15 and 30 mg/day for 56 days) in comparison to placebo in 180 adults with focal epilepsy has been scheduled (NCT05481905).
2.3 ETX-101
ETX-101 can be described as “a non-replicating, recombinant adeno-associated viral vector serotype 9 (rAAV9) comprising a GABAergic regulatory element (reGABA) and an engineered transcription factor that increases transcription of the SCN1A gene (eTFSCN1A)” [34]. The product is intended as a one-time intracerebroventricular (i.c.v.) treatment for patients with Dravet syndrome.
ETX-101 has been designed with the aim to restore the function of inhibitory GABAergic interneurons of patients with Dravet syndrome by upregulating the expression of voltage-gated type I sodium channels (Nav1.1), thus correcting the functional defect underlying the disease. The regulatory element in ETX101 was engineered by interrogating human genetic sequences surrounding the GAD1 gene, expressed specifically in GABAergic interneurons, with the goal of limiting transgene expression to GABAergic inhibitory neurons and reducing off-target expression within excitatory cells [35].
In non-human primates, a one-time unilateral i.c.v. injection was found to be well tolerated and to be associated with widespread distribution of the vector and efficient transgene expression throughout the brain [35, 36]. In a genetic mouse model of Dravet syndrome, a one-time bilateral i.c.v. injection of ETX-101 resulted in increased SCN1A messenger RNA (mRNA) transcripts specifically within GABAergic inhibitory interneurons, and increased NaV1.1 protein levels in the brain. These changes were associated with reduction in spontaneous and hyperthermia-induced seizures, and prolonged survival. The protocol of the phase I/II trial of ETX-101 in infants and children with Dravet syndrome (NCT05419492) is available at ClinicalTrials.gov [34].
2.4 ETX-155
ETX-155 is a novel neuroactive steroid that shares with other neurosteroids an ability to act as a positive allosteric modulator of both synaptic and extrasynaptic GABAA receptors [37]. It is currently being developed for the treatment of focal epilepsy and major depressive disorder [38].
Preclinically, ETX-155 has been reported to be effective in various rodent seizure models, including seizures induced by pentylenetetrazole and maximal electroshock, as well as in animal models of anxiety and depression [37]. In a phase I, single ascending dose study in healthy subjects that evaluated oral doses between 5 and 300 mg, the maximal tolerated dose was found to be 135 mg, with somnolence and dizziness being dose-limiting TEAEs. In multiple-dose studies investigating doses of 60 mg once daily for 7 and 14 days, the most commonly reported TEAEs were somnolence, headache and fatigue [37]. Tolerability was improved when the dose was taken in the evening compared with dosing in the morning.
After single doses of 5–300 mg, ETX-155 is rapidly absorbed from the gastrointestinal tract with Cmax values occurring at 2–4 h after dosing [37]. Within the dose range tested, plasma ETX-155 concentrations increased dose proportionally. Co-administration with food had no clinically meaningful effect on the bioavailability of a 30-mg oral dose, suggesting that ETX-155 may be categorised as a Biopharmaceutics Classification System/Biopharmaceutical Drug Disposition and Classification System Class 1 drug. After multiple dosing, steady state is achieved after 8 days, consistent with a half-life of about 40 h [37]. A preliminary analysis of data from three patients included in a phase Ib proof-of-concept study on the effect of ETX-155 on the photoparoxismal EEG response in patients with photosensitive epilepsy showed inconclusive results, putatively because of plasma drug concentrations being significantly lower than expected [39].
2.5 NRP2945
NRP2945 is an engineered derivative of the neural regeneration peptide (NRP), which belongs to a family of gene-encoded proteins found in mammals and endowed with neuroregenerative and anti-inflammatory properties [40, 41]. NRP2945 is derived from the peptide encoded by the human NRP gene, also known as calcium-dependent activator of protein secretion 2 (CAPS2) and corresponds to the amino acid position 40–50 within the CAPS2 sequence [42, 43]. NRP2945 has been reported to interact with the chemokine receptor with cxc motif 4 and with another chemokine receptor subunit, leading to increased expression of GABAA receptor α and β subunits and upregulation of GABA signalling [41]. Although only a small proportion of circulating NRP2945 crosses the blood–brain barrier, the picomolar levels of the peptide that reach the brain have been deemed to be sufficient for pharmacological activity [43].
In rat studies, NRP2945 (20 μg/kg intraperitoneally) has been found to protect against seizures induced by pentylenetetrazole (50 μg/kg intraperitoneally) [42]. In the same model, NRP2945 inhibited the neuronal damage induced by pentylenetrazole and increased the expression of GABAA receptor α and β subunits and glutamic acid decarboxylase 65-kD isoform in the hippocampus and somatosensory cortex, suggesting that antiseizure and neuroprotective effects were related to increased GABAergic signalling. After a single subcutaneous dose of 20 μg/kg, NRP2945 inibited seizure activity in the GAERS model of absence epilepsy [43]. The reduction in seizure activity in the GAERS persisted for at least 10 days following a 28-day treatment with NRP2945; an effect that was was interpreted as suggestive of a disease-modifying effect [43]. An antiepileptogenic effect has also been reported in the pilocarpine rat model of temporal lobe epilepsy. In this model, treatment with NRP2945 (20 μg/kg, subcutaneously) for 7 days starting 24 h after cessation of pilocarpine-induced status epilepticus reduced the frequency of spontaneous recurrent seizures, but had no effect on seizure frequency when treatment was given after epilepsy had already developed [44]. NRP2945 has also been reported to have activity in models of other diseases, including motor neuron disease, peripheral neuropathy, multiple sclerosis and spinal cord injury, but details of these studies are not available [45].
Clinical studies of NRP2945 have not been published to date. Based on information listed in the sponsor’s website [45], multiple daily doses up to 25 μg/kg were well tolerated in healthy subjects over a 28-day treatment period (one subcutaneous bolus every 48 h). The half-life of NRP2945 was 22–25 min, with Cmax values of 1–1.5 ng/mL and no accumulation over time. An unspecified blood-based biomarker indicated target engagement in the brain. A phase IIa, placebo-controlled, adjunctive-therapy, cross-over trial of NRP2945 has been conducted in Melbourne, Australia, in patients with drug-resistant epilepsy and ‘absence epilepsy EEG patterns’ [45]. The trial apparently provided evidence of a favourable impact of the peptide on seizure activity, but the information provided in the sponsor’s website was insufficient to evaluate the data meaningfully. Further clinical trials are being planned in patients with Lennox–Gastaut syndrome and other types of epilepsy [45].
2.6 NRTX-1001
NRTX-1001 (previously known as NTX-001) is a cryopreserved product that comprises GABAergic post-mitotic interneurons of a specific pallial-type lineage derived from human pluripotent stem cells [46, 47]. The interneurons are designed for direct implantation into the epileptogenic tissue in order to permit release of GABA and suppress seizure activity at the site of the epileptic focus [48].
In the kainate mouse model of chronic mesiotemporal seizures, a single intrahippocampal administration of NRTX-1001 has been found to reduce or suppress electrographic seizures persistently without adverse behavioural effects [47,48,49]. Approximately 75% of epileptic mice treated with NRTX-1001 achieved stable seizure freedom compared with 8% of animals in the control group. Biodistribution studies showed that cell persistence was restricted to the hippocampus [48,49,50]. Metabolic abnormalities identified by magnetic resonance spectroscopy in the epileptic hippocampus partially normalised after administration of NRTX-1001 [50]. The antiseizure effect, which was consistent across different cell lots and studies, was associated with reduced hippocampal neurodegeneration and granule cell dispersion, without evidence of the appearance of ectopic tissues, tumours or teratomas at a histopathological examination 7.5 months after implantation [45, 47]. Preclinical safety studies included magnetic resonance imaging-guided intrahippocampal delivery in immunosuppressed non-human primates using a clinical-grade stereotactic delivery system, and found persistence of NRTX-1001 for at least 3 months post-implantation [50].
NRTX-1001 has recently entered clinical development in patients with drug-resistant unilateral mesial temporal lobe epilepsy. The first-in-human study (NCT05135091) includes an open-label dose escalation in up to ten patients, followed by a randomised trial with inclusion of a sham control group to evaluate safety and efficacy in up to 30 patients [47, 50]. The first two patients treated have shown encouraging results, with seizure counts reduced by > 90% at 6 months and 3 months after implantation, respectively [51].
2.7 STK-001
Dravet syndrome results from a pathogenic SCN1A gene variant that leads to impaired expression of functional voltage-gated type I sodium channels (Nav1.1) in inhibitory GABAergic interneurons [52]. Stimulation of expression of functional SCN1A gene protein in these interneurons to restore GABAergic inhibition provides a rational approach to counteract the manifestations of the disease. STK-001 is an antisense oligonucletide developed by applying the Targeted Augmentation of Nuclear Gene Output (TANGO) technology [53] to specifically activate the functional (wild-type) SCN1A allele in patients with Dravet syndrome, thereby increasing the levels of productive SCN1A mRNA transcript and boosting functional protein levels [20, 54]. Although STK-001 cannot be defined as a GABAergic agent per se, it is discussed here because ultimately its potential therapeutic action is dependent on its ability to improve the function of GABAergic interneurons.
After i.c.v. or intrathecal administration, STK-001 has been found to increase productive mRNA transcripts, and to upregulate brain Nav1.1 protein levels in mice, rats and non-human primates [20]. Intracerebroventricular STK-001 was also effective in increasing SCN1A mRNA levels and NaV1.1 protein expression in a mouse model of Dravet syndrome [55]. In the same model, these effects were associated with restored excitability of parvalbumin interneurons and reduced occurrence of electrographic seizures, together with a prolongation of survival [54, 55].
One phase I/II trial of single and multiple ascending doses of intrathecally administered STK-001 in patients with Dravet syndrome aged 2–18 years (NCT04442295) is ongoing in the USA, together with an associated extension study (NCT04740476). A multiple-dose study is also ongoing in the UK. [56]. The choice of the initial doses was based on a pharmacokinetic model developed with data from non-human primates and published data for animal-human scaling [57]. As of 21 February, 2022, 29 patients had received at least one dose at the 10-mg, 20-mg or 30-mg dose level, and no safety concerns related to the antisense nucleotide had emerged [58]. Dose-dependent increases in plasma STK-001 exposure were observed, and the antisense oligonucleotide was detected in cerebrospinal fluid samples up to 6 months after a single dose [58]. Although preliminary findings suggest an improvement in seizure frequency following STK-001 treatment [56], a full assessment of efficacy data has not been reported to date.
3 Drugs Under Clinical Investigation for Potential Repurposing in Epilepsy
3.1 Alprazolam
Alprazolam is a 1,4-benzodiazepine approved in the USA and other countries for the acute treatment of generalised anxiety disorder in adults, and for the treatment of panic disorder with or without agoraphobia in adults. Its actions are mediated by positive allosteric modulation of GABAA receptors. Like other benzodiazepines, alprazolam is active in animal models of genetic epilepsy and against seizures induced by electrical stimulation and various chemical convulsants [59,60,61,62,63]. It has not generally been used as an ASM, but its potential value in the treatment of seizure disorders has recently been considered as part of a broader effort to explore novel routes of administration for the suppression of prolonged or repetitive seizures.
Currently available rescue seizure treatments using the rectal, buccal or intranasal route utilise compounds such as midazolam and diazepam, which are established ASMs [64]. Alprazolam is being repurposed as an ASM for use by the inhalation route. The Staccato® device delivers alprazolam as an aerosol that, via normal breathing, reaches the lung for rapid uptake into the systemic circulation [65]. In a placebo-controlled, proof-of-concept study in five individuals with photosensitive idiopathic generalised epilepsy, Staccato® alprazolam (S-ALP) at doses of 0.5, 1.0 and 2.0 mg was effective in suppressing the photoparoxismal EEG response, with an onset of activity within 2 min after dosing and persistence of effect through 4 h at the lowest doses and at least 6 h at higher doses [65]. Treatment-emergent adverse events were mild to moderate, appeared to be dose dependent, and consisted in cough, dysgeusia, oral dysesthesia, sedation and somnolence. Mean (± standard deviation) plasma concentrations of alprazolam at 2 min after the 0.5-mg dose were 5.1 ± 2.4 ng/mL, and increased dose proportionally at higher doses. Visual inspection of plasma alprazolam concentration profiles suggested a biphasic decline, with a terminal phase consistent with the drug’s known half-life of 9–16 h [66].
A phase IIb, randomised, double-blind, placebo-controlled, parallel-group, in-patient trial of S-ALP (1.0 and 2.0 mg) has been completed in 163 adults with focal or generalised epilepsy and a predictable pattern of prolonged or repetitive seizures [67]. The primary endpoint was the proportion of responders (response rate), defined as those patients in whom seizure activity ceased within 2 min after administration of the study medication with no recurrence of seizure activity within 2 h. The response rate was identical (65.8%) at S-ALP doses of 1.0 and 2.0 mg, compared with 42.5% for placebo (p < 0.04 for each S-ALP dose vs placebo). Treatment-emergent adverse events were mostly mild to moderate and consisted mainly of cough and somnolence (both 14.5%) and dysgeusia (13.2%), with no clear dose dependency. Plasma alprazolam concentration–time profiles were similar across different seizure types. Overall, these data demonstrate that S-ALP is effective in terminating seizure activity rapidly. Of note, none of the patients had generalised tonic-clonic seizures or focal-to-bilateral-tonic-clonic seizures during the study, and therefore efficacy in terminating this seizure type cannot be inferred. The hypothesis that S-ALP may have a faster onset of action compared with other benzodiazepines given by alternative non-parenteral routes will also require formal head-to-head testing in an ad hoc study [67]. A phase III trial of S-ALP in patients 12 years of age and older with stereotypical prolonged seizures is ongoing (NCT05077904).
3.2 Bumetanide
The rationale for investigating bumetamide as a potential antiseizure drug stems from evidence that certain seizure disorders, most notably neonatal seizures, may be caused by an increased intracellular level of chloride ions (Cl−) in cerebral neurons. Under these conditions, GABA-induced opening of chloride channels leads from an outflow of Cl− from the cytoplasm and a consequent paradoxical depolarising (excitatory) response [68, 69]. The increase in the intracellular Cl− level can result from upregulation of the sodium (Na+)-potassium (K+)-Cl−- transporter isoform 1 (NKCC1), which is involved in Cl− inflow, and/or downregulation of the K+-Cl−-transporter isoform 2 (KCC2), which extrudes Cl− from the cytoplasm [68, 69]. While other mechanisms can play a role in intracellular Cl− homeosthasis [70], inhibitors of NKCC1 such as bumetanide have been considered as potential treatments to reduce intracellular Cl− levels and correct the dysfunction in GABA signalling [71]. Of note, bumetanide inhibits not only NKKC1, but also Na+-K+-Cl−-transporter isoform 2 (NKCC2), which is expressed in the kidney and other organs and plays a key role in the diuretic effects of bumetanide [72]. Several off target effects, some of which might contribute to antiseizure effects, have also been described, including inhibition of carbonic anhydrases, modulation of G protein-coupled receptor 35, inhibition of organic anion transporter 3 and other organic anion transporter transporters, and an interaction of unclear significance with the α3 subunit of the GABAA receptor [72].
Although bumetanide does show antiseizure activity in vitro and in vivo [71, 73], in most seizure models, including those intended to mimic neonatal seizures, its main effect consists of potentiating the antiseizure activity of phenobarbital [20, 68, 74, 75]. Preclinical evidence of seizure protection, however, has not been consistent [76, 77], possibly owing to its poor brain penetration [77]. Concerns have also been expressed about the implications of some of the off-target effects of bumetanide, as well as the consequences of NKCC1 inhibition at non-neuronal sites such as glial cells, the choroid plexus, endocrine cells and immune cells [20]. As a result, the potential value of bumetanide as an ASM, particularly for the treatment of neonatal seizures, has been a subject of intense debate [71, 72, 77, 78].
The results of two recent clinical studies conducted to date have contributed to the controversy. The first of these studies was an open-label, dose-finding, feasibility trial designed to assess the pharmacokinetics, efficacy and safety of bumetanide (0.05–0.3 mg/kg) added on to phenobarbital in full-term neonates with hypoxic ischaemic encephalopathy and electrographic seizures [79]. The trial was stopped prematurely after the enrolment of 14 participants because of the limited evidence of seizure reduction, and the occurrence of hearing impairment in three of 11 surviving infants. However, two of the three infants with hearing impairments had also received aminoglycosides, the ototoxic potential of which can be increased by bumetanide [71]. The second study used a randomised, double-blind, placebo-controlled, dose-escalation design in neonates with an electrographic seizure occurring at least 30 min after a loading dose of phenobarbital (≥ 20 and < 40 mg/kg) [80]. Bumetanide 0.1–0.3 mg/kg (treated group, n = 27) or placebo (control group, n = 16) was administered in conjunction with phenobarbital 5–10 mg/kg, with continuous EEG monitoring from ≥ 2 h before (baseline) to ≥ 48 h after study drug administration (post-treatment). The primary endpoints of the study were the pharmacokinetics and safety of bumetanide, and electrographic seizure burden was included as an exploratory endpoint. Bumetanide was found to have a median half-life of 16.0 h and a median clearance of 0.10 mL/min/kg, with large individual variability. Some of the neonates were also treated with hypothermia, which was associated with lower bumetanide clearance values compared with neonates not exposed to hypothermia. Diuresis as an adverse event was recorded more often in the treated group than in the control group (48% vs 13%, p = 0.02). Four neonates died, one in the treated group and three in the control group. Among survivors, hearing impairment was recorded in 2 of 26 treated neonates, both of whom also received aminoglycosides, versus 0 of 13 controls. After adjustment for seizure burden (which differed markedly between groups), the decrease from baseline in seizure burden from 0 to 4 h and from 2 to 4 h post-treatment was significantly greater among the treated neonates than among controls. Overall, the findings were considered to provide reassuring safety data, and a strong signal of antiseizure efficacy [71]. As mentioned above, the rationale for continuing the clinical development of bumetanide in neonatal seizures is currently being debated [71, 72, 77, 78].
Of note, a bumetanide analogue (NPT 2042), ultimately intended for use as ‘adjunct antiseizure treatment for patients with medically intractable epilepsy’, has recently entered clinical development (NCT05503511), but no information on its structure, pharmacological properties and rationale for development appears to be available [81]. Another compound, IAMA-6, a putatively selective NKCC1 inhibitor targeting epilepsy and other central nervous system disorders as potential indications, is currently in preclinical development [82].
3.3 Ivermectin
Ivermectin is an anthelmintic that exerts its action against parasites by activating glutamate-gated Cl− channels, which are absent in vertebrates [83]. Invermectin, however, also acts as an activator of GABAA receptors by binding to at least two different binding sites distinct from the benzodiazepine binding site. At low (nanomolar) concentrations in vitro, ivermectin enhances GABAA receptor-mediated responses to GABA, and also increases benzodiazepine binding to GABAA receptors, while at higher concentrations it causes a direct activation of the GABAA receptor [84, 85]. As recently reviewed by Löscher [85], ivermectin has many additional actions, including activation of glycine receptors, α7 nicotinic cholinergic receptors and G-protein-gated inwardly rectifying K+ channels. It also acts as a positive allosteric modulator of purinergic adenosine triphosphate-gated P2X4 receptors, as a ligand of farnesoid X receptors [85] and, possibly, as an activator of ATP-sensitive potassium channels and a modulator of the opioidergic system and nitric oxide pathway [86, 87]. Finally, ivermectin has been reported to exert anti-inflammatory actions through a variety of mechanisms [88, 89] and, in particular, to inhibit neuroinflammation in a murine model of experimental autoimmune encephalomyelitis through regulation of T-cell proliferation and function [89]. Some of these properties, particularly those related to inhibition of inflammation, may have implications for seizure control, even though based on available information, the antiseizure effects of ivermectin can be mainly ascribed to actions on the GABAergic system [85, 90, 91].
Interest in evaluating potential effects of ivermectin on seizure susceptibility was stimulated initially by the discovery of its interactions with the GABA system [92]. Antiseizure effects of the drug were reported as early as 1986 in a genetic avian model of photosensitive epilepsy [92]. Since then, protective activity against electrically and chemically induced seizures has been reported in various rodent models [86, 87, 91, 93, 94], including the 6-Hz model in mice and hippocampal kindling in rats [85]. In these models, however, antiseizure effects are generally seen at doses that produce toxicity, which together with other considerations led Löscher [85] to advise against clinical development of the drug for the treatment of epilepsy. Although there have been reports of reduced seizure frequency after ivermectin treatment in patients with onchocerciasis-associated epilepsy, the improvement in these patients may have simply reflected an indirect effect secondary to reduced microfilarial load in the central nervous system [95,96,97]. There has also been a report of improved seizure control in a small group of patients with drug-resistant mostly focal epilepsy treated with ivermectin (10 mg/day once daily three or seven times a week) added on to pre-existing ASMs [98]. Because of the lack of a control group and other methodological limitations, however, the findings of the latter study are difficult to interpret.
After oral administration, peak plasma ivermectin concentrations are usually attained at about 4 h after dosing. The drug is cleared primarily by cytochrome P450 3A-mediated metabolism, with a mean half-life of about 18 h [99]. Ivermectin shows poor penetration across the blood–brain barrier because it is a substrate of the efflux transport P-glycoprotein, which limits access of many molecules that are P-glycoprotein substrates to the brain [85]. Although ivermectin is generally well tolerated at the low doses used in the treatment of onchocerciasis (approximately 150 µg/kg body weight as a single oral dose) [99], concerns have been expressed about potential neurotoxic effects (especially in patients with a dysfunctional blood–brain barrier) at the higher doses (up to 60 mg/day) and prolonged duration of treatment that are currently being tested in patients with epilepsy [85].
A capsule formulation of ivermectin (EQU-001) is currently in development for the treatment of various neurological conditions, including drug-resistant epilepsy [85, 100]. A 12-week, phase II, randomised, double-blind, adjunctive-therapy, placebo-controlled, safety, tolerability and exploratory efficacy study (NCT05063877) has been completed recently. The trial evaluated EQU-001 doses of 10, 20, 40 and 60 mg, administered once daily, in a small group of patients (n = 43) with uncontrolled, mostly focal seizures. According to a press release from the sponsor [100], EQU-001 was found to be safe well tolerated, and no treatment-related serious adverse events were reported. Neurological and psychological adverse events occurred in less than 10% of participants and were similar in the EQU-001 and placebo arms. There was a trend for seizure frequency to improve with increasing EQU-001 doses (after excluding the 40-mg dose), but the study was not powered for efficacy. A reduction in inflammatory biomarkers in the circulation was identified in the 60-mg dose group [100]. A 16-week, randomised, double-blind, placebo-controlled trial (NCT05473442) to assess the antiseizure efficacy of EQU-001 at maintenance doses of 20 and 60 mg/day in adults with uncontrolled focal seizures is currently ongoing.
4 Summary and Conclusions
Although many of the currently marketed ASMs act on the GABA system (Table 1), only three of those, namely vigabatrin, tiagabine and ganaxolone, were rationally designed to exert a GABAergic effect. A fourth rationally designed compound, progabide, was developed in the 1980s as a GABA prodrug but never became established because of disappointing clinical efficacy results and the propensity to cause liver toxicity [101,102,103,104]. Gabapentin was developed as a GABA derivative but it soon became clear that, although it can allosterically modulate GABAA receptors, its primary mode of action is not via GABAergic mechanisms but through interference with voltage-gated calcium channels [105]. All other ASMs listed in Table 1 were discovered through random screening or serendipitous observations, and their interactions with the GABA system became apparent only when their development was already advanced [5].
The last decade saw a renewed interest in targeting the GABA system for the treatment of epilepsy. As discussed in the previous sections of this article, several treatments are now in development that are specifically intended to target GABAergic transmission. A review of their modes of action and pursued indications reveals a remarkable heterogeneity in objectives and approaches (Table 2), which may allow the broad categorisation of these treatments into three groups.
The first group consists of positive allosteric modulators of GABAA receptors. One of these, S-ALP, is a benzodiazepine not used in the past as an ASM, and being now repurposed as a rescue medication for the treatment of prolonged and repetitive seizures. Utilisation of a benzodiazepine for this indication is not a novelty, but the mode of administration of S-ALP by inhalation does represent a first in epilepsy treatment. Whether S-ALP represents an advance over other benzodiazepines currently used for the same indication remains to be determined. It is also unclear whether the inhalation route is applicable to individuals with severe seizure types such as generalised or focal-to-bilateral tonic-clonic seizures. Another set of compounds in this category consists of subunit-selective allosteric modulators. The rationale for developing these drugs, which include the α2/3/5 subtype-selective agents darigabat and ENX-101, builds on evidence that subunit selectivity and, in particular, avoidance of α1 subunit activation, may retain some favourable effects of benzodiazepines, including their antiseizure action, while avoiding or minimising undesirable effects such as cognitive dysfunction, somnolence, tolerance and abuse liability [14, 19, 106,107,108,109,110,111]. Other subunit-selective GABAA receptor allosteric modulators that target seizures as their primary indication have been described, including KRM-II-81 [109, 110] and SAN2219 [112], but to our knowledge they are not yet in clinical development. Among subtype-selective agents, darigabat is the most advanced in development, and has shown promising antiseizure activity in a proof-of-concept trial in photosensitive epilepsy [25]. Larger scale studies, however, are needed to determine whether the promise of subunit-selective GABAA receptor allosteric modulators being superior to benzodiazepines for the maintenance treatment of epilepsy will be fulfilled in the clinical setting. A different approach to GABAA receptor allosteric modulation is the targeting of the neurosteroid modulatory site. ETX-155 is described as a ‘next-generation’ orally active neurosteroid that enhances GABAergic responses at synaptic and extrasynaptic GABAA receptors [38]. Publicly accessible information on this compound is very limited, and it is unclear how it differs from other neurosteroids such as ganaxolone [6]. This question is especially relevant because ETX-155 is apparently being targeted for the treatment of focal epilepsy [38], an indication that was also pursued with ganaxolone with disappointing results [6, 113]. Similar considerations apply to LPCN 2101, another orally administered neurosteroid scheduled for a proof-of-concept study in patients with photosensitive epilepsy, although the indication to be pursued for this compound are as yet unclear [114, 115].
A second group of investigational compounds consists of two marketed non-central nervous system drugs, namely bumetanide and ivermectin, that have been considered for repurposing in the treatment of seizure disorders. These agents differ in pharmacological properties as well as indications being pursued. Bumetanide is a NKCC1 inhibitor investigated for the treatment of phenobarbital-resistant neonatal seizures based on evidence that increased intracellular levels of chloride in the immature brain can result in GABA having a paradoxical depolarising effect. Ivermectin, in contrast, has a very complex pharmacology. It has many other mechanisms of action in addition to those involving the GABA system and is being investigated in epilepsy mainly based on the results of preclinical studies in experimental seizure models. One common feature of bumetanide and ivermectin is that the rationale for pursuing their clinical development in seizure disorders has come recently under criticism [71, 72, 77, 85]. Apparently, no additional clinical studies are currently scheduled for bumetanide in neonatal seizures, whereas a randomised controlled trial of ivermectin in patients with uncontrolled focal seizures is ongoing (NCT05473442).
The third and most innovative group of treatments includes cell and gene therapies, as well as treatments to regulate gene expression. Intracerebral implantation of GABAergic interneurons (NRTX-001) is being evaluated in patients with mesial temporal lobe epilepsy, whereas an antisense oligonucleotide (STK-001) to restore the function of GABAergic interneurons by upregulating NaV1.1 sodium channels is under investigation in patients with Dravet syndrome. A clinical trial of gene therapy using an adenoviral vector (ETX-101) is scheduled for initiation. Some of these treatments could theoretically prove to be game changers, but it would be premature to speculate on their value prior to completion of safety and efficacy studies. The same applies to NRP2945, a subcutaneously administered neuroactive peptide that reportedly upregulates the expression of GABAA receptor α and β subunits.
Overall, the current pipeline of investigational treatments demonstrates that targeting the GABA system remains an attractive approach in epilepsy drug development. Although this article focused on investigational treatments for which clinical data are available, or likely to be available in the short term, preclinical research in this area is also very active. Drug candidates being investigated preclinically include, among others, novel GABAA receptor agonists and allosteric modulators [14, 33, 111], compounds targeting GABAB receptors [116, 117], a highly potent inhibitor of GABA transaminase [118], a new class of selective NKCC1 inhibitors [82] and novel GABA-targeting viral vectors [119]. At the other extreme of the development spectrum, clinical studies are ongoing to assess novel applications of already marketed GABA-targeting ASMs. Notable examples include the investigation of vigabatrin as an antiepileptogenic and disease-modifying agent in infants with tuberous sclerosis complex [120], and the evaluation of low-dose valproic acid delivered by continuous i.c.v. infusion as a long-term treatment for patients with drug-resistant epilepsy [121, 122].
References
Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN. Revisiting the role of neurotransmitters in epilepsy: an updated review. Life Sci. 2021;265: 118826.
Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl. 3):8–12.
Liu YQ, Yu F, Liu WH, He XH, Peng BW. Dysfunction of hippocampal interneurons in epilepsy. Neurosci Bull. 2014;30:985–94.
Bryson A, Reid C, Petrou S. Fundamental neurochemistry review: GABAA receptor neurotransmission and epilepsy: principles, disease mechanisms and pharmacotherapy. J Neurochem. 2023;165(1):6–28.
Löscher W. Single-target versus multi-target drugs versus combinations of drugs with multiple targets: preclinical and clinical evidence for the treatment or prevention of epilepsy. Front Pharmacol. 2021;12: 730257.
Perucca E, Bialer M, White HS. New GABA-targeting therapies for the treatment of seizures and epilepsy: I. Role of GABA as modulator of seizure activity and recently approved medications acting on the GABA system. CNS Drugs. https://doi.org/10.1007/s40263-023-01027-2.
Perucca E, Brodie MJ, Kwan P, Tomson T. 30 years of second-generation antiseizure medications: impact and future perspectives. Lancet Neurol. 2020;19:544–56.
Perucca E, Perucca P, White HS, Wirrell EC. Drug resistance in epilepsy. Lancet Neurol. 2023;22:723–34.
Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1398–442.
Zuberi SM, Wirrell E, Yozawitz E, Wilmshurst JM, Specchio N, Riney K, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1349–97.
Shaimardanova AA, Chulpanova DS, Mullagulova AI, Afawi Z, Gamirova RG, Solovyeva VV, et al. Gene and cell therapy for epilepsy: a mini review. Front Mol Neurosci. 2022;15: 868531.
Owen RM, Blakemore D, Cao L, Flanagan N, Fish R, Gibson KR, et al. Design and identification of a novel, functionally subtype selective GABAA positive allosteric modulator (PF-06372865). J Med Chem. 2019;62:5773–96.
Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8.
Cerne R, Lippa A, Poe MM, Smith JL, Jin X, Ping X, Golani LK, et al. GABAkines: advances in the discovery, development, and commercialization of positive allosteric modulators of positive allosteric modulators of GABAA. Pharmacol Ther. 2022;234:10830.
Engin E. GABAA receptor subtypes and benzodiazepine use, misuse, and abuse. Front Psychiatry. 2023;13:1060949.
Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–4.
Zeilhofer HU, Möhler H, Di Lio A. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists. Trends Pharmacol Sci. 2009;30:397–402.
Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800.
Nickolls SA, Gurrell R, van Amerongen G, Kammonen J, Cao L, Brown AR, et al. Pharmacology in translation: the preclinical and early clinical profile of the novel α2/3 functionally selective GABAA receptor positive allosteric modulator PF-06372865. Br J Pharmacol. 2018;175:708–25.
Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Perucca P, et al. Progress report on new antiepileptic drugs: a summary of the Sixteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XVI): II. Drugs in more advanced clinical development. Epilepsia. 2022;63:2883–910.
Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Perucca P, et al. Progress report on new antiepileptic drugs: a summary of the Fifteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XV). II. Drugs in more advanced clinical development. Epilepsia. 2020;61:2365–85.
Gurrell R, Iredale P, Evrard A, Duveau V, Ruggiero C, Roucard C. Pronounced antiseizure activity of the subtype-selective GABAA positive allosteric modulator darigabat in a mouse model of drug-resistant focal epilepsy. CNS Neurosci Ther. 2022;28:1875–82.
Duveau V, Buhl DL, Evrard A, Ruggiero C, Mandé-Niedergang B, Roucard C, et al. Pronounced antiepileptic activity of the subtype-selective GABAA-positive allosteric modulator PF-06372865 in the GAERS absence epilepsy model. CNS Neurosci Ther. 2019;25:255–60.
Gurrell R, Whitlock M, Wei H, Shen Z, Ogden A. Safety, tolerability, and pharmacokinetics of multiple repeated oral doses of the α2/3/5-subtype selective GABAA-positive allosteric modulator PF-06372865 in healthy volunteers. Clin Pharmacol Drug Dev. 2021;10:756–64.
Gurrell R, Gorman D, Whitlock M, Ogden A, Reynolds DS, DiVentura B, et al. Photosensitive epilepsy: robust clinical efficacy of a selective GABA potentiator. Neurology. 2019;92:e1785–95.
Cerevel. Darigabat. www.cerevel.com/. Accessed 9 Mar 2023.
Simen A, Whitlock M, Qiu R, Miceli J, Zumpano L, Du Metz M, et al. An 8-week, randomized, phase 2, double-blind, sequential parallel-group comparison study of two dose levels of the GABAA positive allosteric modulator PF-06372865 compared with placebo as an adjunctive treatment in outpatients with inadequate response to standard of care for generalized anxiety disorder. J Clin Psychopharmacol. 2019;39:20–7.
Gurrell R, Dua P, Feng G, Sudworth M, Whitlock M, Reynolds DS, et al. A randomised, placebo-controlled clinical trial with the α2/3/5 subunit selective GABAA positive allosteric modulator PF-06372865 in patients with chronic low back pain. Pain. 2018;159:1742–51.
Engrail Therapeutics. Press release, July 7, 2022. https://www.businesswire.com/news/home/20220607005544/en/Engrail-Therapeutics-Announces-Positive-Results-of-ENX-101-Phase-1b-Clinical-Study-and-Prepares-for-Initiation-of-ENACT-Phase-2-Trial-in-Focal-Epilepsy. Accessed 25 Feb 2023.
Engrail Therapeutics. ENX-101. https://www.engrail.com/enx-101/. Accessed 25 Feb 2023.
Vinkers CH, Olivier B. Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective GABA(A) receptor modulators? Adv Pharmacol Sci. 2012;2012: 416864.
Castellano D, Shepard RD, Lu W. Looking for novelty in an “old” receptor: recent advances toward our understanding of GABAARs and their implications in receptor pharmacology. Front Neurosci. 2021;14: 616298.
Janković SM, Dješević M, Janković SV. Experimental GABAA receptor agonists and allosteric modulators for the treatment of focal epilepsy. J Exp Pharmacol. 2021;13:235–44.
ClinicalTrials.gov. A clinical study to evaluate the safety and efficacy of ETX101 in infants and children with SCN1A-positive Dravet syndrome (ENDEAVOR), June 15, 2022. https://clinicaltrials.gov/ct2/show/NCT05419492. Accessed 29 Feb 2023.
Tanenhaus A, Stowe T, Young A, McLaughlin J, Aeran R, Lin IW, et al. Cell-selective adeno-associated virus-mediated SCN1A gene regulation therapy rescues mortality and seizure phenotypes in a Dravet syndrome mouse model and is well tolerated in nonhuman primates. Hum Gene Ther. 2022;33:579–97.
Belle A, Lin W, McLaughlin J, Li J, Lucey G, Soe M et al. ETX101, a GABAergic interneuron selective AAV-mediated gene therapy for the treatment of SCN1A+ Dravet syndrome: biodistribution and safety in non-human primates. (abstract). Annual Meeting of the American Epilepsy Society (virtual meeting), December 4-8, 2020. https://www.aesnet.org/education/annual-meeting/aes-abstract-search#?wst=da0320f7f221f5870d4395dbb4df063b. Accessed 26 Feb 2023.
Versavel M. ETX-155, A novel GABAA positive allosteric modulator: Eliem Therapeutics, Inc. Presentation given at the 2022 Epilepsy Foundation Pipeline Conference, Santa Clara, Ca, June 5-6, 2022. https://www.youtube.com/watch?v=JuzSYOx-kGk. Accessed 6 Mar 2023.
Eliem Therapeutics. Pipeline. https://eliemtx.com/pipeline/. Accessed 7 Mar 2023.
Eliem Therapeutics. Eliem provides update on ETX-810 and ETC-155 clinical programs. Press release, April 25, 2022. https://ir.eliemtx.com/node/6921/pdf. Accessed 7 Mar 2023.
Gorba T, Bradoo P, Antonic A, Marvin K, Liu DX, Lobie PE, et al. Neural regeneration protein is a novel chemoattractive and neuronal survival-promoting factor. Exp Cell Res. 2006;312:3060–74.
Sieg F. Mini-review of neural regeneration peptide in brain development. J Stem Cell Res Ther. 2016;1:147–8.
Sajadian A, Esteghamat S, Karimzadeh F, Eshaghabadi A, Sieg F, Speckmann EJ, et al. Anticonvulsant effect of neural regeneration peptide 2945 on pentylenetetrazol-induced seizures in rats. Neuropeptides. 2015;49:15–23.
Dezsi G, Sieg F, Thomas M, O’Brien TJ, Van der Hart M, Jones NC. Disease-modifying effects of neural regeneration peptide 2945 in the GAERS model of absence epilepsy. Neurochem Res. 2017;42:2055–64.
Lovisari F, Guarino A, Soukupova M, Falcicchia C, Ingusci S, Marino P, et al. Anti-epileptogenic effect of NRP2945 in the pilocarpine model of temporal lobe epilepsy. Eur J Pharmacol. 2021;901: 174068.
CuroNZ. Research overview-discovery-pipeline-pharmacodynamics-clinical studies. http://www.curonz.com/research.html. Accessed 26 Feb 2023.
Hampel P, Parekh, M, Kim H, Sevilla ES, Porkka F, Vogel A et al. NRTX-1001: human inhibitory neuron cell therapy suppresses seizures and reduces histopathology in mouse model of chronic epilepsy with high repeatability across multiple studies and manufacturing lots. (abstract). Annual Meeting of the American Epilepsy Society, Nashville, TN, December 2-6, 2022. https://www.aesnet.org/education/annual-meeting/aes-abstract-search#?wst=acb8577691a4550b8138b209a5c093b4. Accessed 26 Feb 2023.
Nicholas CR. NRTX-1001: inhibitory neuron cell therapy for drug resistant TLE. Presentation given at the 2022 Epilepsy Foundation Pipeline Conference, Santa Clara, Ca, June 5-6, 2022. https://www.youtube.com/watch?v=UuNRuy6hL2g. Accessed 28 Mar 2023.
Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Perucca P, et al. Progress report on new antiepileptic drugs: a summary of the Fifteenth Eilat Conference New Antiepileptic Drugs and Devices (EILAT XV). I. Drugs in preclinical and early clinical development. Epilepsia. 2020;61:2340–64.
Priest C, Parekh M, Blankenberger W, Hampel P, Sevilla ES, Traver D, et al. Preclinical development of NRTX-1001, an inhibitory interneuron cellular therapeutic for the treatment of chronic focal epilepsy. Annual Meeting of the American Epilepsy Society, Chicago, IL, December 3-7, 2021 [abstract]. https://www.aesnet.org/education/annual-meeting/aes-abstract-search#?wst=e2f6b92ed83e2e317e0f9027f8a70041. Accessed 26 Feb 2023.
Blum D, Parekh M, Hampel P, Kim H, Sevilla E, Adler A, et al. NRTX-1001: first-in-class human inhibitory neuron cell therapy for phase I/II clinical investigation in chronic focal epilepsy (abstract). Annual Meeting of the American Epilepsy Society, Nashville, TN, December 2-6, 2022. https://www.aesnet.org/education/annual-meeting/aes-abstract-search#?wst=acb8577691a4550b8138b209a5c093b4. Accessed 26 Feb 2023.
Neurona Therapeutics. Press release, February 15, 2023. https://www.neuronatherapeutics.com/news/press-releases/021423/. Accessed 26 Feb 2023.
Catterall WA. Dravet syndrome: a sodium channel interneuronopathy. Curr Opin Physiol. 2018;2:42–50.
Lim KH, Han Z, Jeon HY, Kach J, Jing E, Weyn-Vanhentenryck S, et al. Antisense oligonucleotide modulation of non-productive alternative splicing upregulates gene expression. Nat Commun. 2020;11:3501.
Wengert ER, Wagley PK, Strohm SM, Reza N, Wenker IC, Gaykema RP, et al. Targeted augmentation of nuclear gene output (TANGO) of Scn1a rescues parvalbumin interneuron excitability and reduces seizures in a mouse model of Dravet Syndrome. Brain Res. 2022;1775: 147743.
Han Z, Chen C, Christiansen A, Ji S, Lin Q, Anumonwo C, et al. Antisense oligonucleotides increase Scn1a expression and reduce seizures and SUDEP incidence in a mouse model of Dravet syndrome. Sci Transl Med. 2020;12:558.
Bryson S. Stoke’s Phase 3 trial of STK-001 for Dravet planned for next year. Commentary posted January 10, 2023. https://dravetsyndromenews.com/news/. Accessed 28 Mar 2023.
Meena M, Ticho B, Barriere O, Gosselin N. A pharmacokinetic (PK) model for STK-001, an antisense oligonucleotide (ASO), based on data from non-human primates (NHP) enables dose selection in patients with Dravet syndrome (DS). Annual Meeting of the American Epilepsy Society, Chicago, IL, December 3-7, 2021. https://www.aesnet.org/education/annual-meeting/aes-abstract-search#?wst=2514d0f818db5d8605793417f23604c8. Accessed 27 Feb 2023.
Laux L, Sullivan J, Roberts C, Schreiber J, Scott P, Devinsky O, et al. MONARCH interim analyses: a phase 1/2a US study investigating safety and drug exposure of STK-001, an antisense oligonucleotide (ASO), in children and adolescents with Dravet syndrome (DS). (abstract). Annual Meeting of the American Epilepsy Society, Nashville, TN, December 2-6, 2022. https://www.aesnet.org/education/annual-meeting/aes-abstract-search#?wst=f19a31b6712a527e0592cba339a1b561. Accessed 27 Feb 2023.
Jenck F, Moreau JL, Bonetti EP, Martin JR, Haefely WE. Ro 19–8022, a nonbenzodiazepine partial agonist at benzodiazepine receptors: neuropharmacological profile of a potential anxiolytic. J Pharmacol Exp Ther. 1992;262:1121–7.
Giusti P, Ducić I, Puia G, Arban R, Walser A, Guidotti A, Costa E. Imidazenil: a new partial positive allosteric modulator of gamma-aminobutyric acid (GABA) action at GABAA receptors. J Pharmacol Exp Ther. 1993;266:1018–28.
De Sarro G, Gitto R, Rizzo M, Zappia M, De Sarro A. 1,4-Benzodiazepine derivatives as anticonvulsant agents in DBA/2 mice. Gen Pharmacol. 1996;27:935–41.
Herink J. Effect of alprazolam and ketamine on seizures induced by two different convulsants. Acta Medica (Hradec Kralove). 1997;40:9–11.
Qian B, Sun Y, Wu Z, Wan L, Chen L, Kong S, et al. Epileptiform response of CA1 neurones to convulsant stimulation by cyclothiazide, kainic acid and pentylenetetrazol in anaesthetized rats. Seizure. 2011;20:312–9.
Maglalang PD, Rautiola D, Siegel RA, Fine JM, Hanson LR, Coles LD, et al. Rescue therapies for seizure emergencies: new modes of administration. Epilepsia. 2018;59(Suppl. 2):207–15.
French JA, Wechsler R, Gelfand MA, Pollard JR, Vazquez B, Friedman D, et al. Inhaled alprazolam rapidly suppresses epileptic activity in photosensitive participants. Epilepsia. 2019;60:1602–9.
Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam: therapeutic implications. Clin Pharmacokinet. 1993;24:453–71.
French J, Biton V, Dave H, Detyniecki K, Gelfand MA, Gong H, et al. A randomized phase 2b efficacy study in patients with seizure episodes with a predictable pattern using Staccato® alprazolam for rapid seizure termination. Epilepsia. 2023;64:374–85.
Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13.
Savardi A, Borgogno M, De Vivo M, Cancedda L. Pharmacological tools to target NKCC1 in brain disorders. Trends Pharmacol Sci. 2021;42:1009–34.
Glykys J, Dzhala V, Egawa K, Kahle KT, Delpire E, Staley K. Chloride dysregulation, seizures, and cerebral edema: a relationship with therapeutic potential. Trends Neurosci. 2017;40:276–94.
Staley KJ. Clarifications regarding bumetanide for neonatal seizures. Epilepsia. 2022;63:1863–7.
Löscher W, Kaila K. CNS pharmacology of NKCC1 inhibitors. Neuropharmacology. 2022;205: 108910.
Mazarati A, Shin D, Sankar R. Bumetanide inhibits rapid kindling in neonatal rats. Epilepsia. 2009;50:2117–22.
Cleary RT, Sun H, Huynh T, Manning SM, Li Y, Rotenberg A, et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS ONE. 2013;8: e57148.
Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63:222–35.
Johne M, Römermann K, Hampel P, Gailus B, Theilmann W, Ala-Kurikka T, et al. Phenobarbital and midazolam suppress neonatal seizures in a non-invasive rat model of birth asphyxia while bumetanide is ineffective. Epilepsia. 2021;62:920–34.
Kaila K, Löscher W. Bumetanide for neonatal seizures: no light in the pharmacokinetic/dynamic tunnel. Epilepsia. 2022;63:1868–73.
Stafstrom CE. Don’t get BUM’d out: bumetanide may yet prove beneficial for neonatal seizures. Epilepsy Curr. 2021;21:341–3.
Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an ope-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14:469–77.
Soul JS, Bergin AM, Stopp C, Hayes B, Singh A, Fortuno CR, et al. A pilot randomized, controlled, double-blind trial of bumetanide to treat neonatal seizures. Ann Neurol. 2021;89:327–40.
ClinicalTrials.gov. Safety and pharmacokinetic study of NPT 2042 soft-gelatin capsules administered orally to healthy adult subjects. https://clinicaltrials.gov/ct2/show/NCT05503511. Accessed 27 Mar 2023.
Savardi A, Borgogno M, De Vivo M, Cancedda L. Selective NKCC1 inhibitors for the treatment of neurodevelopmental and neurological disorders with defective NKCC1/KCC2 expression-ratio [abstract]. Neurology. 2023;100(17 Suppl 2 2):P2-12.003.
Hibbs RE, Gouaux E. Principles of activation and permeation of an anion-selective Cys-loop receptor. Nature. 2011;474:54–60.
Estrada-Mondragon A, Lynch JW. Functional characterization of ivermectin binding sites in α1β2γ2L GABA(A) receptors. Front Mol Neurosci. 2015;8:55.
Löscher W. Is the antiparasitic drug ivermectin a suitable candidate for the treatment of epilepsy? Epilepsia. 2023;64:553–66.
Jourian S, Rahimi M, Manavi MA, Pahlevan-Fallahy MT, Mohammad Jafari R, Amini A, et al. Possible interaction of opioidergic and nitrergic pathways in the anticonvulsant effect of ivermectin on pentylenetetrazole-induced clonic seizures in mice. Neurochem Res. 2023;48:885–94.
Manavi MA, Mohammad Jafari R, Shafaroodi H, Ejtemaei-Mehr S, Sharifzadeh M, Dehpour AR. Anticonvulsant effects of ivermectin on pentylenetetrazole- and maximal electroshock-induced seizures in mice: the role of GABAergic system and KATP channels. Heliyon. 2022;8: e11375.
Mathachan SR, Sardana K, Khurana A. Current use of ivermectin in dermatology, tropical Medicine, and COVID-19: an update on pharmacology, uses, proven and varied proposed mechanistic action. Indian Dermatol Online J. 2021;12:500–14.
Xie Y, Jin C, Sang H, Liu W, Wang J. Ivermectin protects against experimental autoimmune encephalomyelitis in mice by modulating the Th17/Treg balance involved in the IL-2/STAT5 pathway. Inflammation. 2023;1–13. https://doi.org/10.1007/s10753-023-01829-y.
Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, et al. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 2000;295:1051–60.
De Souza S, Stilck SRAN, Bernardi MM. Possible anxiolytic effects of ivermectin in rats. Vet Res Commun. 2002;26:309–21.
Crichlow EC, Mishra PR, Crawford RD. Anticonvulsant effects of ivermectin in genetically-epileptic chickens. Neuropharmacology. 1986;25:1085–8.
Mayer TW, Horton ML. Modulation of monomethylhydrazine-induced seizures by ivermectin. Toxicol Lett. 1991;57:167–73.
Trailović SM, Varagić VM. The effect of ivermectin on convulsions in rats produced by lidocaine and strychnine. Vet Res Commun. 2007;31:863–7.
Kipp W, Burnham G, Kamugisha J. Improvement in seizures after ivermectin. Lancet. 1992;340:789–90.
Dusabimana A, Tsebeni Wafula S, Raimon SJ, Fodjo JNS, Bhwana D, Tepage F, et al. Effect of ivermectin treatment on the frequency of seizures in persons with epilepsy infected with onchocerca volvulus. Pathogens. 2020;10:21.
Mandro M, Siewe Fodjo JN, Dusabimana A, Mukendi D, Haesendonckx S, Lokonda R, et al. Single versus multiple dose ivermectin regimen in onchocerciasis-infected persons with epilepsy treated with phenobarbital: a randomized clinical trial in the Democratic Republic of Congo. Pathogens. 2020;9:205.
Diazgranados-Sanchez JA, Mejia-Fernandez JL, Chan-Guevara LS, Valencia-Artunduaga MH, Costa JL. Ivermectina como coadyuvante en la epilepsia refractaria. Rev Neurol. 2017;65:303–10.
Stromectol (ivermectin tablets). Prescribing information (2009). https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050742s026lbl.pdf. Accessed 22 Feb 2023.
Equilibre Biopharmaceuticals. Equilibre Biopharmaceuticals announces positive topline results from phase 2 clinical trial of EQU-001 (NCT05063877) for safety, tolerability and preliminary efficacy as adjunctive therapy for focal seizures in adults with epilepsy. Press release, January 30, 2023. https://eqneuro.com/news-publications/. Accessed 20 Jun 2023.
Dam M, Gram L, Philbert A, Hansen BS, Lyon BB, Christensen JM, et al. Progabide: a controlled trial in partial epilepsy. Epilepsia. 1983;24:127–34.
Schmidt D. Progabide as an add-on drug for epilepsy refractory to high dose antiepileptic drug therapy. Neurosci Lett. 1984;29(47):357–60.
Crawford P, Chadwick D. A comparative study of progabide, valproate, and placebo as add-on therapy in patients with refractory epilepsy. J Neurol Neurosurg Psychiatry. 1986;49:1251–7.
Leppik IE, Dreifuss FE, Porter R, Bowman T, Santilli N, Jacobs M, et al. A controlled study of progabide in partial seizures: methodology and results. Neurology. 1987;37:963–8.
Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–62.
Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at a1 and a2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16.
Atack JR. GABAA receptor subtype-selective modulators. I. a2/a3-selective agonists as non-sedating anxiolytics. Curr Top Med Chem. 2011;11:1176–202.
Zuiker RGJA, Chen X, Østerberg O, Mirza NR, Muglia P, de Kam M, et al. NS11821, a partial subtype-selective GABAA agonist, elicits selective effects on the central nervous system in randomized controlled trial with healthy subjects. J Psychopharmacol. 2016;30:253–62.
Witkin M, Lippa A, Smith JL, Jin X, Ping X, Biggerstaff A, et al. The α2,3-selective potentiator of GABAA receptors, KRM-II-81, reduces nociceptive-associated behavior induced by formalin and spinal nerve ligation in rats. Pharmacol Biochem Behav. 2019;180:22–31.
Witkin JM, Lippa A, Smith JL, Jin X, Ping X, Biggerstaff A, et al. The imidazodiazepine, KRM-II-81: an example of a newly emerging generation of GABAkines for neurological nd psychiatric disorders. Pharmacol Biochem Behav. 2022;213: 173321.
Pandey KP, Divović B, Rashid F, Golani LK, Cerne R, Zahn NM, et al. Structural analogs of the GABAkine KRM-II-81 are orally bioavailable anticonvulsants without sedation. J Pharmacol Exp Ther. 2023;385:50–61.
Saniona selects SAN2219 as preclinical candidate for epilepsy. Press release, December 20, 2022. https://saniona.com/newsroom/. Accessed 27 Mar 2023.
Lappalainen J, Tsai J, Amerine W, Patroneva A. A multicenter, double-blind, randomized, placebo-controlled phase 3 trial to determine the efficacy and safety of ganaxolone as adjunctive therapy for adults with drug-resistant focal-onset seizures (P5237). Neurology. 2017;88:P5.237.
Lipocine. FDA clears LPCN 2101 IND for adult epilepsy treatment. Press release, July 11, 2022. https://ir.lipocine.com/press-releases. Accessed 28 Mar 2023.
Chern CR, Lauková M, Schonwald A, Kudová E, Chodounská H, Chern CJ, et al. Novel neurosteroid pregnanolone pyroglutamate suppresses neurotoxicity syndrome induced by tetramethylenedisulfotetramine but is ineffective in a rodent model of infantile spasms. Pharmacol Rep. 2023;75:177–88.
Avoli M, Lévesque M. GABAB receptors: are they missing in action in focal epilepsy research? Curr Neuropharmacol. 2022;20:1704–16.
Wang P, Nan S, Zhang Y, Fan J. Effects of GABAB receptor positive allosteric modulator BHF177 and IRS-1 on apoptosis of hippocampal neurons in rats with refractory epilepsy via the PI3K/Akt pathway. Cell Biol Int. 2022;46:1775–8.
Feja M, Meller S, Deking LS, Kaczmarek E, During MJ, Silverman RB, et al. OV329, a novel highly potent γ-aminobutyric acid aminotransferase inactivator, induces pronounced anticonvulsant effects in the pentylenetetrazole seizure threshold test and in amygdala-kindled rats. Epilepsia. 2021;62:3091–104.
Niibori Y, Duba-Kiss R, Bruder JT, Smith JB, Hampson DR. In silico prediction and in vivo testing of promoters targeting GABAergic inhibitory neurons. Mol Ther Methods Clin Dev. 2023;28:330–43.
Kotulska K, Kwiatkowski DJ, Curatolo P, Weschke B, Riney K, Jansen F, et al. Prevention of epilepsy in infants with tuberous sclerosis complex in the EPISTOP trial. Ann Neurol. 2021;89:304–14.
Cook M, Murphy M, Bulluss K, et al. Anti-seizure therapy with a long-term, implanted intra-cerebroventricular delivery system for drug-resistant epilepsy: a first-in-man study. E Clin Med. 2020;22: 100326.
Distag E. Intraventricular therapy for epilepsy. Presentation given at the 2022 Epilepsy Foundation Pipeline Conference, Santa Clara, Ca, June 5-6, 2022. https://www.youtube.com/watch?v=86j5oC_g7yQ. Accessed 28 Mar 2023.
Riban V, Heulard I, Reversat L, Si Hocine H, Verleye M. Stiripentol inhibits spike-and-wave discharges in animal models of absence seizures: a new mechanism of action involving T-type calcium channels. Epilepsia. 2022;63:1200–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The preparation of this article was not supported by any funding source.
Conflicts of Interest/Competing Interests
Emilio Perucca received speaker fees or fees from consulting or participation in advisory boards/data safety monitoring boards from Angelini, Arvelle, Biopas, Eisai, GW Pharma, Janssen, PMI Life Sciences, Sanofi group of companies, Shackelford Pharma, SKL Life Science, Sun Pharma, Takeda, UCB Pharma, Xenon Pharma and Zogenix, and royalties from Wiley, Elsevier and Wolters Kluwers, all outside the submitted work. Meir Bialer received consultancy fees from Boehringer Ingelheim, Clexio Therapeutics, Guidepoint, Pharma2B, Shackelford Pharma and US WorldMeds. H. Steve White has received grant funding from UCB Pharma and Eisai Pharmaceuticals, Neurelis, Inc. and consultant fees from GW Pharmaceuticals, Neurelis, Inc., Takeda Pharmaceuticals, SK Life Sciences and Jazz Pharmaceuticals and speaker honoraria from SK Pharmaceuticals, Takeda Pharmaceuticals and UCB Pharma.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
This article is an invited review. All authors contributed to the literature search. EP and MB produced the original draft. HSW revised critically the sections related to the mechanisms of action and pharmacological properties of the compounds listed, and contributed to the drafting of Sect. 4. All authors participated equally in the finalisation of the manuscript and they all met ICMJE criteria for authorship. All authors have read and approved the final submitted manuscript, and agree to be accountable for the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Perucca, E., White, H.S. & Bialer, M. New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: II. Treatments in Clinical Development. CNS Drugs 37, 781–795 (2023). https://doi.org/10.1007/s40263-023-01025-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01025-4