Abstract
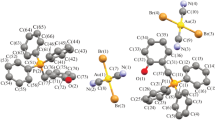
The reactions of potassium dichlorodicyanoaurate with n-propyl-, iso-butyl-, and n-heptyltriphenylphosphonium bromides in water followed by recrystallization from acetonitrile give dichlorodicyanoaurate complexes [Ph3P(n-Pr)][Au(CN)2Cl2] (I), [Ph3P(i‑Bu)][Au(CN)2Cl2] (II), and [Ph3P(n-Hp)][Au(CN)2Cl2] (III). Compounds I−III are identified by elemental analysis and IR spectroscopy. The structure of compound III is also proved by X-ray diffraction (XRD) (CIF file CCDC no. 2094701). According to the XRD data, complex III consists of n-heptyltriphenylphosphonium cations and crystallographically independent planar square dichlorodicyanoaurate anions of two types with similar geometric parameters. The steric organization of the crystal of complex III is formed by hydrogen bonds С–H∙∙∙N≡C (2.55–2.63 Å) and С–H∙∙∙Cl–Au (2.87 Å). In addition, the structure contains С–H∙∙∙π(Ph) contacts with the distances from the hydrogen atom to the benzene ring plane equal to 2.80 Å.


Similar content being viewed by others
REFERENCES
Kumar, K., Stefanczyk, O., Chorazy, S., et al., Inorg. Chem., 2019, vol. 58, p. 5677. https://doi.org/10.1021/acs.inorgchem.8b03634
Nicholas, A.D., Bullard, R.M., Pike, R.D., and Patterson, H., Eur. J. Inorg. Chem., 2019, vol. 2019, p. 956. https://doi.org/10.1002/ejic.201801407
Belyaev, A., Eskelinen, T., Dau, T.M., et al., Chem. Eur. J., 2017, vol. 24, p. 1404. https://doi.org/10.1002/chem.201704642
Katz, M.J., Ramnial, T., Yu, H., and Leznoff, D.B., J. Am. Chem. Soc., 2008, vol. 130, p. 10662. https://doi.org/10.1021/ja801773p
Ovens, J.S., Christensen, P.R., and Leznoff, D.B., Chem. Eur. J., 2016, vol. 22, p. 8234. https://doi.org/10.1002/chem.201505075
Ovens, J.S., Geisheimer, A.R., Bokov, A.A., et al., In-org. Chem., 2010, vol. 49, p. 9609. https://doi.org/10.1021/ic101357y
Katz, M.J. and Leznoff, D.B., J. Am. Chem. Soc., 2009, vol. 131, p. 18435. https://doi.org/10.1021/ja907519c
Thompson, J.R., Goodman-Rendall, K.A.S., and Leznoff, D.B., Polyhedron, 2016, vol. 108, p. 93. https://doi.org/10.1016/j.poly.2015.12.026
Thompson, J.R., Katz, M.J., Williams, V.E., and Leznoff, D.B., Inorg. Chem., 2015, vol. 54, p. 6462. https://doi.org/10.1021/acs.inorgchem.5b00749
Lefebvre, J., Batchelor, R.J., and Leznoff, D.B., J. Am. Chem. Soc., 2004, vol. 126, p. 16117. https://doi.org/10.1021/ja049069n
Lefebvre, J., Korcok, J.L., Katz, M.J., and Leznoff, D.B., Sensors, 2012, vol. 12, p. 3669. https://doi.org/10.3390/s120303669
Varju, B.R., Ovens, J.S., and Leznoff, D.B., Chem. Commun., 2017, no. 48, p. 6500. https://doi.org/10.1039/c7cc03428h
Ovens, J.S. and Leznoff, D.B., Chem. Mater., 2015, vol. 27, p. 1465. https://doi.org/10.1021/cm502998w
Ovens, J.S. and Leznoff, D.B., Inorg. Chem., 2017, vol. 56, p. 7332. https://doi.org/10.1021/acs.inorgchem.6b03153
Ovens, J.S. and Leznoff, D.B., CrystEngComm, 2018, vol. 20, p. 1769. https://doi.org/10.1039/c7ce02167d
Lefebvre, J., Chartrand, D., and Leznoff, D.B., Polyhedron, 2007, vol. 26, p. 2189. https://doi.org/10.1016/j.poly.2006.10.045
Geisheimer, A.R., Huang, W., Pacradouni, V., et al., Dalton Trans., 2011, vol. 40, p. 7505. https://doi.org/10.1039/c0dt01546f
Lefebvre, J., Callaghan, F., Katz, M.J., et al., Chem. Eur. J., 2006, vol. 12, p. 6748. https://doi.org/10.1002/chem.200600303
Lefebvre, J., Chartrand, D., and Leznoff, D.B., Inorg. Chem., 2009, vol. 48, p. 55. https://doi.org/10.1002/chem.200600303
Shevchenko, D.P. and Khabina, A.E., Bull. South Ural State Univ., Ser. Chem., 2021, vol. 13, p. 58. https://doi.org/10.14529/chem210106
Efremov, A.N., Sharutin, V.V., Sharutina, O.K., et al., Russ. J. Chem. Chem. Tech., 2020, vol. 63, p. 10. https://doi.org/10.6060/ivkkt.20206303.6097
Sharutin, V.V., Sharutina, O.K., Tarasova, N.M., et al., Russ. Chem. Bull., 2020, vol. 69, p. 1892. https://doi.org/10.1007/s11172-020-2975-4
Sharutin, V.V., Bull. South Ural State Univer., Ser. Chem., 2020, vol. 12, p. 74. https://doi.org/10.14529/chem200208
Sharutin, V.V., Sharutina, O.K., Tarasova, N.M., and Efremov, A.N., Russ. J. Inorg. Chem., 2020, vol. 65, p. 169. https://doi.org/10.1134/s0036023620020151
Sharutin, V.V., Sharutina, O.K., Efremov, A.N., and Eltsov, O.S., Russ. J. Coord. Chem., 2020, vol. 46, p. 631. https://doi.org/10.1134/s1070328420090031
SMART. SAINT-Plus. Versions 5.0. Data Collection, Processing Software for the SMART System, Madison: Bruker AXS Inc., 1998.
SHELXTL/PC, Versions 5.10, An Integrated System for Solving, Refining, Displaying Crystal Structures from Diffraction Data, Madison: Bruker AXS Inc., 1998.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339. https://doi.org/10.1107/s0021889808042726
Cordero, B., Gómez, V., Platero-Prats, A.E., et al., Dalton Trans., 2008, p. 2832. https://doi.org/10.1039/b801115j
Mantina, M., Chamberlin, A.C., Valero, R., et al., J. Phys. Chem. A, 2009, vol. 113, p. 5806. https://doi.org/10.1021/jp8111556
Nishio, M., Phys. Chem. Chem. Phys., 2011, p. 13873. https://doi.org/10.1039/c1cp20404a
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Shevchenko, D.P., Khabina, A.E. Organyltriphenylphosphonium Dichlorodicyanoaurates [Ph3PR][Au(CN)2Cl2] (R = n-Pr, i-Bu, and n-Hp): Synthesis and Structures. Russ J Coord Chem 49, 521–525 (2023). https://doi.org/10.1134/S1070328423600328
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600328