Abstract
Intercommunity (lethal) aggression is a familiar component of the behavioural repertoire of many forest-dwelling chimpanzee (Pan troglodytes) communities. However, until now, the absence of intercommunity attacks – including killings – in communities that live in open, mosaic environments has supported hypotheses of reduced resource competition in drier habitats, and informed referential models of early hominin social dynamics in a similar habitat. In June 2020, we observed the first instance of intercommunity lethal aggression, a male-committed infanticide, by the Issa chimpanzee community, which live in a savannah-mosaic habitat in the Issa Valley, western Tanzania. The carcass was recovered by researchers after it was abandoned by the attackers. Here, we give a detailed account of the events leading up to and including the infanticide, and contextualise our observations with what has been described for other chimpanzee communities. Notably, in contrast to the majority of reported intercommunity infanticides, the infant male victim was castrated (and not cannibalised), making this the youngest reported castration. This observation of intercommunity aggression disproves its hypothesised absence in savannah-dwelling chimpanzees, which by extension, has implications for early hominin evolution. We suggest that the near absence of observations of intercommunity aggression in savannah chimpanzee communities is most likely due to the lack of long-term study communities, and in some cases geographic isolation. We hypothesise that food-rich areas within a habitat with otherwise widely distributed food sources may select for intense intercommunity aggression despite the low population density characteristic of savannah communities. Anecdotes such as this add to the comparative database available on intercommunity killings in chimpanzee society, improving our ability to draw inferences about their evolutionary significance.
Similar content being viewed by others
Introduction
Comparisons between chimpanzee and human behaviour are an important method to model the social dynamics of the human–chimpanzee last common ancestor (LCA) and early members of the human lineage (hominins) (e.g. Muller 2017; Wilson and Glowacki 2017; Wrangham and Benenson 2017). Notably, wild chimpanzee and human hunter–gatherer groups exhibit similar rates of intergroup lethal aggression (Wrangham et al. 2006), but we cannot know whether similarities were inherited from the LCA or evolved convergently (Wilson and Glowacki 2017). Chimpanzees that live in habitats analogous to those reconstructed for early hominins – mosaic environments that are more open and arid than evergreen forests (van Leeuwen et al. 2020) – are suggested to make particularly informative models for early hominin behaviour due to shared ecology (Moore 1992, 1996; Hunt and McGrew 2002; Hernandez-Aguilar et al. 2007; Pruetz and Bertolani 2009; Pruetz and Herzog 2017; Lindshield et al. 2021; Hunt et al. 2021; Drummond-Clarke et al. 2022). Below, we report the first observation of an intercommunity lethal encounter in a savannah community of chimpanzees and discuss it in the context of our current understanding of chimpanzee lethal aggression.
Chimpanzee (Pan troglodytes) social dynamics are characterised by intra- and intercommunity hostility. Notably, intra- and intercommunity infant killing (hereafter ‘infanticide') have been documented since early observations of wild chimpanzees began (e.g. Suzuki 1971; Bygott 1972; Goodall 1977), and adult males are widely known to cooperate to conduct territorial patrols and kill strangers from neighbouring communities (Goodall 1979; Boesch and Boesch-Achermann 2000; Watts and Mitani 2001; Wilson and Wrangham 2003; Watts et al. 2006; Muller and Mitani 2005). Currently, intercommunity lethal aggression has been observed, or inferred, at eight different communities and in three of four subspecies (or rather, all subspecies with long-term observations), with male-committed intercommunity infanticide being particularly prevalent (Wilson et al. 2014).
The evolutionary origins of male-committed intercommunity lethal aggression in chimpanzees remains a key question in palaeoanthropology, with many hypotheses existing that are centred around adaptive strategies that increase male fitness, directly and indirectly, by improving access to resources – primarily food and females (e.g. Wrangham 1979; Wrangham and Smuts 1980; Wilson et al. 2014). The ‘exploitation hypothesis’ suggests that perpetrators increase fitness directly by gaining nutritional benefits from consuming the victim (Hrdy 1979); however, inconsistency in consumption rates of victims make this an unlikely motive for intercommunity killings (Kirchhoff et al. 2018). The ‘sexual selection hypothesis’ (Hrdy 1979; Nishida et al. 1979; Arcadi and Wrangham 1999), where the killing of an unrelated infant shortens postpartum subfecundity in the victim’s mother, would directly increase reproductive opportunity of a male attacker. In the case of intercommunity infanticides, the female could also be coerced to leave her own community and join the attacking group to improve the safety of her next infant (the ‘female coercion hypothesis’; Arcadi and Wrangham 1999). However, uncertainty about mating rates between male attackers and mothers of infanticide victims from other communities makes such hypotheses of direct sexual selection hard to test (e.g. Boesch et al. 2008). Alternatively, the ‘resource competition’ or ‘range expansion hypothesis’ suggests that intercommunity aggression deters extra-community members from border areas, leading to range expansion of the attacking group (Williams et al. 2004). This is hypothesised to increase male fitness indirectly through expanding the area where females in their own community can forage safely, thereby increasing resident female fitness and the potential for their own (male) reproductive success (Pusey 2001; Watts et al. 2002; Williams et al. 2004), and is supported by observations of decreased inter-birth intervals of resident females with range expansion at Gombe (Williams et al. 2004; Pusey et al. 2005). A larger home range is also hypothesised to have the secondary effect of increased immigration of new females (Watts and Mitani 2000; Watts et al. 2002; Williams et al. 2004; Sherrow and Amsler 2007).
When the attacker(s) and victim are male (as is the majority in reported intercommunity attacks; Wilson et al. 2014), rival reduction may also explain killings (e.g. Arcadi and Wrangham 1999; Newton-Fisher 1999; Kutsukake and Matsusaka 2002; Watts et al. 2002). A link to male rival reduction may also be supported by a high frequency of castration of adolescent/adult male victims in intercommunity attacks, if we can assume this indicates knowledge of the role of the testes in reproduction (e.g. Wilson et al. 2015).
In parallel to the above hypotheses, the ‘imbalance of power hypothesis’ predicts the likelihood of a violent encounter increases when one party greatly outnumbers the other (or victim’s) party (Manson and Wrangham 1991; Wrangham 1999), and has support from observations of intercommunity violence across multiple sites (Sherrow and Amsler (2007) and references there-in).
Despite high variability in mortality rates caused by intercommunity killings across forest-dwelling communities (see Table 3 in Boesch et al. 2008), until now, intercommunity killings have never been documented in a savannah-dwelling community. Of the few chimpanzee studies conducted in arid, open habitats, most have been short term and/or not fully habituated (e.g. Mt Assirik and Semliki; Hunt and McGrew 2002; Hunt 2020), or the study community is suggested to be geographically isolated from neighbouring communities (e.g. Fongoli; Pruetz et al. 2017), posing obvious limitations on the observation or occurrence of intercommunity interactions of any kind. However, it is notable that savannah chimpanzee communities are characterised by larger home ranges with more sparsely distributed food sources than their forest dwelling counterparts (Moore 1992; Lindshield et al. 2021). Combined with population density being a significant predictor of lethal aggression rates (increased density = increased aggression; Wilson et al. 2014), this lack of observations in savannah-dwelling communities offers support for early hypotheses related to the costs to social organisation in savannah chimpanzees (sensu Moore 1992, 1996), including predictions that lower population density leads to less territoriality in savannah-mosaic communities (e.g. Samson and Hunt 2014). If so, we would expect overlapping ranges, combined with far less, or an absence of, intercommunity aggression.
Here, we report the first observed intercommunity killing between chimpanzees in a savannah-mosaic habitat in the Issa Valley, western Tanzania, and along with it the youngest ever recorded castration in chimpanzee intercommunity aggression. We contextualise the timeline and observations with previously described intercommunity killings and discuss the implications for chimpanzee territoriality and human evolution.
Methods
Study site and subjects
The Issa study site is situated in western Tanzania in the Greater Mahale Ecosystem. It constitutes ~85 km2 comprised of five major valleys separated by steep mountains and flat plateaus ranging from 1050 to 1750 m in elevation (Giuliano et al. 2022). The region is classed as a savannah-mosaic landscape, dominated by miombo woodlands (Brachystegia and Julbernardia spp.) interspersed with rocky outcrops, riparian forest and patches of seasonally inundated grassland (Piel et al. 2017; Drummond-Clarke et al. 2022). Issa is one of the driest and most seasonal habitats for chimpanzees (van Leeuwen et al. 2020; Lindshield et al. 2021). The wet season (April–October) includes nearly all annual rainfall (rainfall averages 1205 mm/year, range 717–1747 ml from 2009 to 2021), with the dry season (monthly rainfall < 100 mm) lasting over 6 months from May to October, marked by annual grass fires predominantly set by humans that burn more than 75% of the landscape (Piel and Stewart unpublished data).
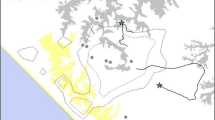
There has been a continuous research presence at Issa since 2008, with the Issa community considered fully habituated (nest-to-nest follows) as of mid-2018. At the time of the current observation, the community consisted of 27 individuals (seven adult males, eight adult females, three adolescent males, two adolescent females, four juveniles, three infants). For this study, infants were classified as unweaned individuals estimated between 0 and 4 years old (yo), juveniles 5–9 yo, adolescents 10–15 yo and adults 16 yo and over or after first pregnancy for females (we estimated all ages). The current home range is estimated at a minimum of 39 km2 (Piel and Stewart, unpublished data; Fig. 1).
Location of the intercommunity fatal encounter (yellow cross) in relation to the research station (pink triangle) and the habituated Issa community’s known home range (black lines). Valleys (dark green strips) are lined with riparian forest, the plateaus are dominated by miombo woodland. The infanticide took place at the intersect of Issa Valley (long valley in north south orientation) and Mgumu Valley (shorter connecting valley). The attacking party remained within 800 m of the attack site for at least 3 h after the attack took place, resuming normal foraging and grooming behaviour
Data collection
The first author and a field assistant (hereafter referred to as ‘the researchers’) conducted a focal follow (Altmann 1974) on juvenile male MO, beginning at 07:05 h on the morning of 4 June 2020. At the time, research teams collected focal and group scan data for long-term research into chimpanzee’s behaviour, diet and ranging patterns. They also collected ad libitum video recording (using Sony FDR-AX53 camcorder) whenever possible of unusual behaviours, in this case the intercommunity encounter, allowing for an accurate timeline and detailed account. The researchers followed the chimpanzee party until 12:30 h. The carcass was retrieved at 14:00 h after being abandoned by the party, and was returned to the station where it was photographed and buried for later retrieval of the skeleton. The age of the infant victim was estimated from its size and dependency on its mother. An ethogram of observed behaviours is provided in Table S1.
Description of intercommunity lethal encounter, 4 June 2020
06:20h: The researchers arrived at the nest site of eight males (four adults, three adolescents and one juvenile; Table 1), in the periphery of the known Issa community home range (Fig. 1), with the entire party still in their nests.
07:10 h: The party, led by IM, began pant-hooting and ran into a riparian forest strip.
07:20 h: The entire party fed on fruit in a Cordia sp. tree.
07:34 h: The party changed feeding trees to a Ficus sp.
08:00 h: The party left the tree and began walking north quickly and quietly. Adolescent male SA was the last to leave the tree, and walked separately from the party, staying behind in the forest, but moving in the same direction. After around 5 min, multiple individuals vocalised, and were answered by SA, who was about 30 m behind the main party. At this point, researchers were behind with SA (since they could not bypass him on the thin forest path), and could not see the main party.
08:09 h: Following the vocalisations, the researchers left SA and found the other males in the woodland, adjacent to the forest strip, where the males had encountered an unidentified adult female and infant. Five males (IM, SAM, WI, DH, MO) were in a tree and two (KIT and WA) were beneath them in the grass, with all individuals vocalising (pant–hoots, barks and screams). The (arboreal) males abruptly descended, and the researchers could hear the screams of an infant chimpanzee. The researchers could see constant displays. After a few minutes, SA pant–hooted from the forest strip, and multiple adult males in the party replied, then IM, WA and KIT ran in the direction of SA’s pant–hoot, returned quickly with SA. Vocalisations and displaying continued for 15 min, with SAM, WI and DH watching the others from several metres above in a nearby tree.
08:27 h: WA climbed up a nearby tree, and the researchers saw he was holding an infant chimpanzee (estimated to be between 2 and 3 yo) against his chest, with the infant’s hand in WA’s mouth (Supplementary video 1). DH and WI followed WA mid-way up the tree.
08:28 h: WA was joined by SAM, SA and IM, with DH and an unknown female (the infant’s mother) just below them in the tree. SA, IM and KIT displayed, SA and WA bit the infant, and SA bit the infant’s penis and scrotum off (Supplementary video 2). The infant was screaming throughout. The infant’s mother attempted to retrieve the infant repeatedly, reaching for its legs before and after the castration, but she was always deterred by KIT who poked the female while WA and SA push her back, and displays by IM. At this point the researchers noticed the female had a swollen, bruised eye, as well as a fresh cut on her buttocks. WA climbed higher in the tree, continuing to hold the infant by the hand in his mouth, while the female barked as the males screamed and hooted. Adolescent WI also climbed up the tree and briefly touched the infant. As WA climbed the tree further, the infant grabbed hold of a branch. WA pulled the infant forcing him to let go of the branch.
08:29 h: SA climbed and sat next to WA, as they renewed attacks on the infant, SA focused on the limbs, biting, pulling, and bending, while WA bit the infant’s abdomen. IM then displaced SA, but did not engage with the (still conscious, but severely wounded) infant. KIT approached and grabbed one of the infant’s feet, while WA held the infant and bit his pelvic area and then the throat. Throughout this period, there were screams, hoots and barks, and the female was not visible but could be heard barking from the ground (Supplementary video 2).
08:30 h: SAM joined the attacking group, bringing the attacking party to five (SAM, SA, WA, IM, KIT). SA and WA bit the infant’s head, while WI bit the infant’s foot and SAM the back. No chewing was observed. IM began hooting and displayed towards WI, who retreated. At this point, weak screams from the infant and barks, likely coming from the female below, could still be heard. IM displaced SAM and targeted the legs/genital area/stomach. WA, SA, IM and KIT continued to bite and tug the infant until his limbs were limp, at this point researchers assumed the infant was dead (Supplementary video 2).
08:31 h: The female (who had climbed up an adjacent tree and back into view) climbed down (passing MO who was sitting just below), and moved out of site on the ground, hooting and barking as she left. After this point, she was not seen again (Supplementary video 3). During this time, KIT, IM, SAM, SA and WA were clustered in the tree, SA and WA still holding on to the body. SA bit and pulled on the limbs, while WA appeared to bite the genital area. KIT attempted to take the carcass from WA but failed, while SA and WA continued to bite it. MO sat separately (approx. 8 m) from the older males, but watched the males and carcass.
08:34 h: WA still held the body, but SA, SAM and KIT continued to pull limbs and appeared to bite it, and IM bit the genital area, but did not try to take the carcass from WA or stop others interacting with it (Supplementary video 3).
08:37 h: KIT took the body from WA, and began to groom the thigh, as WA peered at him (Supplementary video 3).
08:38 h: WA took back hold of the torso and KIT, IM, SA and SA each held part of the carcass. SA bit the right hand, IM bit the genital area, and WA, SA, SAM and KIT continued smelling and biting the carcass (Supplementary video 3).
08:41 h: KIT pried the carcass from WA and began climbing the tree. SAM, WA, IM and SA followed. SAM and WA tried to interact with the carcass, and WA briefly succeeded and appeared to bite it, then left and climbed further up the tree (Supplementary video 3).
08:42 h: IM started a pant-hoot, and others joined in to chorus, during which SAM and KIT moved away from IM. After a few seconds the pant–hooting stopped, and KIT moved further along the branch with the infant’s body, followed by SAM and WA. SAM looked at and touched the castration area, and WA the upper body. IM and SA also approached and held the carcass. IM, SA and WA continued to bite and inspect the hands, upper body and genital area while KIT held on to the carcass (Supplementary video 3).
08:44 h: KIT still had hold of the legs, and SA, IM and WA were still biting the upper body and pelvic area. WI then approached from below, appeared to bite down once on the infant and then retreated. DH and MO sat below the other males in the same tree, but were not showing interest in the scene (Supplementary video 4).
08:45 h: IM moved away and the carcass dropped to the ground from their location around 8 m high in the tree). The first to follow and retrieve the carcass was KIT, with WA, IM and SAM following. DH and MO remained in the tree, watching the others on the ground (Supplementary video 4).
08:46 h: KIT climbed back up the tree with the carcass, followed by SAM, WA, DH and SA. KIT stopped and sat, and MO approached KIT and peered at the carcass, then moved away. After 45 s, KIT resumed climbing with the carcass followed by WA, IM, SAM, DH and SA. WA and IM moved close to KIT and the carcass. WI followed from approx. 4 m (Supplementary video 5).
09:00 h: KIT moved a metre away from the others with the carcass and made and sat in a nest, letting the carcass dangle by the foot (Fig. 2; Supplementary videos 6, 7). WA moved closer to KIT, and dangled his arm next to the carcass but did not touch it (Fig. 2).
Adult, adolescent and juvenile males from the attacking party with the infant’s carcass shortly after the attack. KIT is in a nest made minutes before by him, holding the infant’s carcass (dangling below). WA is showing interest in the carcass but does not take it back from KIT after this point. Screenshot taken from Supplementary video 7 (see Supplementary videos 6 and 7 for nest-making sequence)
09:03 h: KIT left his nest and climbed down the tree, stopping at the same level as MO on another branch. WI moved to KIT and grabbed the pelvis and brought his face to the carcass to bite or smell the area. After a few seconds KIT moved the carcass away from WI, but WI moved closer in and put his lips to the genital area, but did not bite, then moved away to just below KIT and was joined by MO (Supplementary video 8).
09:04 h: KIT swung down, leaving the carcass in the tree above. KIT then reached for a limb of the carcass, and pulled it down, then climbed to the ground dragging the carcass. WI and MO followed. All other chimpanzees were already on the ground (Supplementary video 8).
09:11 h: The party was travelling cardinal direction through the riverine forest strip for about 100 m. KIT dragged the carcass along the ground behind him (Supplementary video 9).
09:18 h: The party stopped, and after a quiet pause, began displaying, leaving the carcass and crossing over a stream. When they returned to the original spot where the carcass remained, still displaying, they were accompanied by two other community members, BO (adult male) and KU (juvenile male), who had not previously been seen that day. The party continued displaying all together for a few minutes– pant–hooting, running, buttress root drumming – then went calm. BO briefly inspected the carcass, looking and smelling, then BO groomed with the other adults. KU, MO, DH and WI examined the carcass on the forest floor.
09:30 h: The party travelled south, leaving the carcass behind. They passed the location where the infanticide had taken place but did not appear to react. At this point, the researchers briefly inspected the infanticide location, which was easily distinguishable by the flattened grass, and saw a small pool of blood on the grass.
The researchers stayed with the party until 12:30 h while they regularly groomed, rested and fed on fruit as they continued to travel south along a valley towards the centre of their known home range.
Although we cannot completely rule out consumption of any kind (i.e. of missing genitals), during all above-described events we did not observe any targeted and seemingly intentional feeding for nutritional purposes, and apart from severe wounds (gashes to the head, neck and torso, lacerations to the hands, legs, and feet, and the missing genitalia, see Fig. 3), the infant’s body was otherwise intact upon retrieval by the researchers that afternoon.
Post-mortem photos of the injuries incurred by the attacking males on the infant victim during the intercommunity lethal encounter. Injuries incurred include: Broken right femur and tibia (A), removed genitalia (A, B), punctured and lacerated throat and chest (A, B), a large gash in the left ribs (C), ripped skin from the left foot and deep wounds on the right hip (D), a deep laceration to the face below the left eye (E), deep wound (to the bone) on the right hand, and ripped skin on the fingers (F)
Discussion
Our observation of intercommunity lethal aggression in the Issa Valley is the first to be observed in a savannah-mosaic community of chimpanzees. Although more data are needed on overall intercommunity encounter rates in savannah-mosaic chimpanzees, this observation falsifies the hypothesis that intercommunity killing does not occur in an open and dry, or low population density context (Samson and Hunt 2014; Wilson et al. 2014).
Comparison with previous reports of lethal intercommunity encounters
Here, a party of eight males participated in an infanticide involving the castration of an infant male (estimated 2–3 yo) from a neighbouring community following an attack on the infant and his likely mother, in a peripheral area of the Issa community’s known home range (Fig. 1). To our knowledge, they did not cannibalise the dead infant, although they maintained control of the carcass for around 1 h after the victim’s death, and remained in the area to forage, rest and groom. The mother eventually left the attacking group and infant’s carcass without any serious injuries. This case of lethal intercommunity aggression bears resemblance to multiple cases already described in forest communities in several ways.
First, as with most intercommunity aggressions, the attack took place in the periphery of the attacking community’s known home range, and the attacking party (eight males, of which seven were adult or adolescent) greatly outnumbered the victims (two individuals, one adult female and her infant), resulting in a ratio very similar to the 8:1 median of adult and adolescent individuals in each party calculated from other attacks (Wilson et al. 2014; Wilson and Glowacki 2017). Additionally, the mixed age range of the all-male group of attackers was consistent with the party composition of cases reported at Taï (Boesch et al. 2008), Mahale (Kutsukake and Matsusaka 2002), and Ngogo (Watts and Mitani 2000). Second, the victims were a lone female and her unweaned dependent, the infant being the focus of the attack as is the case in just over half of observed intercommunity killings (Wilson et al. 2014; Wilson and Glowacki 2017) and further, the infant was male; males account for over 78% of reported intercommunity aggression fatalities (Wilson et al. 2014). Despite these similarities between the current and published accounts, differences exist.
Foremost, this case differed from many intercommunity lethal attacks on sexually immature individuals in that the victim was castrated (Fig. 3A, B). Although commonly described for attacks on sexually mature males (e.g. Wilson et al. 2015), there is only one other reported possible castration of an infant during an intercommunity attack, observed in the Ngogo community (Uganda; Watts and Mitani 2000). However, the sex of the infant was unknown and it was completely cannibalised by the attackers, making castration uncertain (Watts and Mitani 2000). As such, our observation represents the youngest confirmed castration (without cannibalisation) by chimpanzees. Second, although the victim suffered substantial bite wounds and castration, the attackers were not observed to cannibalise the body (also confirmed by the carcass being complete, except the missing genitals, on retrieval). While cannibalism is rare in intercommunity attacks on adult victims, it is common on infant victims (including older infants between 1.5 and 3 yo), especially when the attackers remain where the encounter took place (Arcadi and Wrangham 1999; Kirchoff et al. 2018). Finally, in most cases of chimpanzee intercommunity aggression, the attacking party flee after the victim is wounded or dead (Watts et al. 2002). In the current case, however, the attacking party remained within 800 m of the attack for over 3 h, one of the males even making a day nest to sit in with the carcass at the site of the attack (Fig. 2). We cannot be sure why in some cases attackers retreat and in others they remain, but given the potential for intercommunity aggression, it is likely that no males from the neighbouring community were nearby, so there was minimal threat of (counter-) attack (e.g., Watts et al. 2002). It is also possible that researcher presence deterred non-habituated chimpanzees from approaching (e.g. Tutin and Fernandez 1991; Bertolani and Boesch 2008).
Adaptive strategy
More observations of intercommunity encounters are clearly needed to test hypotheses about selective pressures for intercommunity lethal aggression. While this observation falsifies the hypothesis that intercommunity killing by chimpanzees occurs only in high-density forest settings, without more observations we cannot know whether rates of intercommunity aggression are similar at Issa compared with other chimpanzee communities. However, we can compare this observation with other published cases to assess whether there could be support for previously proposed hypotheses. This observation does not support the exploitation hypothesis (Hrdy 1979), as the victim was not consumed after death. It does however lend support to several different hypotheses.
First, it supports the ‘range expansion hypothesis’ as it took place (i) on the periphery of a (known) home range where researchers have observed members of the neighbouring chimpanzee community (unpublished data) and (ii) in a forest strip with multiple known feeding trees (Cordia sp. and Ficus sp.), during a period of seasonal food scarcity (Wilson and Glowacki 2017; Giuliano et al. 2022). Second, that the attacking party greatly outnumbered the victims supports the ‘imbalance of power hypothesis’, where the larger the attacking party, the lower the cost of attack (Manson and Wrangham 1991; Sherrow and Amsler 2007). Third, that the victim was male, and castrated early in the attack lends support to the ‘male–male competition’ (or rival reduction) hypothesis (Arcadi and Wrangham 1999; Newton-Fisher 1999; Kutsukake and Matsusaka 2002; Watts et al. 2002). Further, castrating the male victim, despite his sexual immaturity, supports the ‘resource defence’ and ‘rival reduction’ hypothesis in that infanticide is functionally equivalent to the killing of adult males (Wilson et al. 2004), where castration is common during intercommunity aggression (e.g. Boesch et al. 2007; Wilson et al. 2015). Although the killing of an infant by males from another community may lend support to the ‘sexual selection’ or ‘female coercion hypothesis’, it is difficult to comment on this without future observation of the female’s ranging and mating patterns. It should however be noted that the female has not been seen with the habituated (attacking) community since the infanticide took place (3 years at the time of writing), providing no evidence that she transferred to the attacking community as a result of the infanticide/coercion.
Implications for chimpanzee behavioural ecology in a savannah-mosaic landscape
There is substantial variation in intercommunity encounter and lethal aggression rates across chimpanzees (Boesch et al. 2008; Wilson et al. 2014). Issa’s single observation of intercommunity lethal aggression after 4 years of behavioural follows is not a low rate compared to other long-term chimpanzee study sites, despite a low population density (Issa rate = 0.25 observed killings/year versus a reported median of 0.24 (min = 0, max = 1.83), calculated from Table 3 in Boesch et al. 2008). This holds even when considering only eastern chimpanzee communities, which are considered more aggressive than the western subspecies based on observations of forest-dwelling communities (Boesch et al. 2008; Wilson et al. 2014). Savannah-mosaic chimpanzees are characterised by ecological temporal and spatial heterogeneity with key foods overall more sporadic, but also more condensed (principally in riparian forest strips) during food scarce times of the year (van Leeuwen et al. 2020; Lindshield et al. 2021). Specifically at Issa during the food scarce period that this intercommunity encounter took place, chimpanzees were observed feeding principally on Cordia and Ficus spp. fruit, both of which occur in forest and have high nutritional value (Piel et al. 2017; Giuliano et al. 2022). Abundant supplies of food in border areas increase the likelihood of intercommunity encounters in forest settings by attracting chimpanzees from both sides of the boundary (Wilson et al. 2012). We therefore hypothesise that increased food sporadicity characteristic of dry habitats could further increase the likelihood of intercommunity encounters (and competition) at high-quality, food-rich patches (e.g. forest strips), counter-balancing negative effects of low population density on encounter rates during times of food scarcity.
Chimpanzees from the Fongoli community (Senegal) are the only other fully habituated savannah-dwelling chimpanzees with long-term observations. Despite 15 years of study at Fongoli, there have been no descriptions of intercommunity encounters. Importantly, Issa and Fongoli differ in taxonomy: Issa Valley hosts the eastern subspecies (P. t. schweinfurthii) and Fongoli the western subspecies (P. t. verus). Although observations of intercommunity interactions in western chimpanzees are largely limited to Taï Forest (due to Taï being the only western site with long-term observations and neighbouring communities), eastern chimpanzees have been described as less cohesive than their western counterparts, and this has been linked to higher rates of intercommunity aggression reported in the eastern subspecies (i.e. smaller parties are more vulnerable to attack, in relation to the imbalance of power hypothesis; Wrangham 1999; Boesch et al. 2008; Wilson et al. 2014). However, in these open habitats individuals from both communities exhibit comparatively high gregariousness in grouping behaviour (Pruetz and Bertolani 2009; Giuliano et al. 2022). This suggests that differences in intercommunity aggression rates between eastern and western communities cannot be linked to cohesiveness alone, forcing us to reconsider the roles of taxonomy and habitat on chimpanzee behaviour. It is notable that environmental differences exist between Issa and Fongoli that may affect intercommunity encounter rates. In particular, the Fongoli community is suggested to be geographically isolated from neighbouring communities by anthropogenic (a paved road and settlement) and natural (Gambia River) features (Pruetz et al. 2017). This not only imposes obvious limitations on the occurrence of intercommunity encounters (and thus aggression) at Fongoli, but is markedly in contrast to the Issa community, for which camera trap footage and field observations (including the encounter reported here) reveal at least one neighbouring community, and likely two (unpublished data).
More data are needed on seasonal ranging patterns and intercommunity encounter rates at Issa (and other savannah-mosaic habitat chimpanzee sites) to further test the role of food rich patches on counterbalancing negative effects of a low population density on encounter rates. Our observations could however lend support to intense competition in food rich patches in a savannah habitat, in that it took place in a forest strip with abundant fruiting trees during a time of food scarcity at Issa (Piel et al. 2017; Giuliano et al. 2022). More observations of intercommunity encounters will be critical in understanding the differences in intercommunity group dynamics between chimpanzees across their ecological range.
Data availability
All data used in this study are included in the published article and its supplementary information files.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267. https://doi.org/10.1163/156853974x00534
Arcadi A, Wrangham RW (1999) Infanticide in chimpanzees: review of cases and a new within-group observation from the Kanyawara study group in Kibale National Park. Primates 40:337–351. https://doi.org/10.1007/BF02557557
Bertolani P, Boesch C (2008) Habituation of wild chimpanzees (Pan troglodytes) of the south group at Taï Forest, Côte d’Ivoire: empirical measure of progress. Folia Primatol 79:162–171. https://doi.org/10.1159/000111720
Boesch C, Boesch-Achermann H (2000) The chimpanzees of Taï Forest. Oxford University Press, Oxford UK
Boesch C, Head J, Tagg N et al (2007) Fatal chimpanzee attack in Loango National Park, Gabon. Int J Primatol 28:1025–1034. https://doi.org/10.1007/s10764-007-9201-1
Boesch C, Crockford C, Herbinger I et al (2008) Intergroup conflicts among chimpanzees in Taï National Park: lethal violence and the female perspective. Am J Primatol 70:519–532. https://doi.org/10.1002/ajp.20524
Bygott JD (1972) Cannibalism among wild chimpanzees. Nature 238:410–411. https://doi.org/10.1038/238410a0
Drummond-Clarke RC, Kivell TL, Sarringhaus L, et al (2022) Wild chimpanzee behavior suggests that a savanna-mosaic habitat did not support the emergence of hominin terrestrial bipedalism. Sci Adv 8:eadd9752. https://doi.org/10.1126/sciadv.add9752
Giuliano C, Stewart FA, Piel AK (2022) Chimpanzee (Pan troglodytes schweinfurthii) grouping patterns in an open and dry savanna landscape, Issa Valley, western Tanzania. J Hum Evol 163:103137. https://doi.org/10.1016/j.jhevol.2021.103137
Goodall J (1977) Infant killing and cannibalism in free-living chimpanzees. Folia Primatol 28:259–282. https://doi.org/10.1159/000155817
Goodall J (1979) Intercommunity interactions in the chimpanzee population of the Gombe National Park. In: Hamburg DA, McCown ER (eds) The great apes. Benjamin/Cummings, Menlo Park, CA, pp 13–53
Hernandez-Aguilar RA, Moore J, Pickering TR (2007) Savanna chimpanzees use tools to harvest the underground storage organs of plants. PNAS 104:19210–19213. https://doi.org/10.1073/pnas.0707929104
Hrdy SB (1979) Infanticide among animals: A review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40. https://doi.org/10.1016/0162-3095(79)90004-9
Hunt KD (2020) Chimpanzee: Lessons from our sister species. Cambridge Univ Press, Cambridge, UK
Hunt KD, McGrew WC (2002) Chimpanzees in the dry habitats of Assirik, Senegal and Semliki Wildlife Reserve, Uganda. In: Boesch C, Hohmann G, Marchant L (eds) Behavioral diversity in chimpanzees and bonobos. Cambridge Univ Press, Cambridge, UK, pp 35–51
Hunt KD, Dunevant SE, Yohler RM, Carlson KJ (2021) Femoral bicondylar angles among dry-habitat chimpanzees (Pan troglodytes schweinfurthii) resemble those of humans: Implications for knee function, australopith sexual dimorphism, and the evolution of bipedalism. J Anthropol Res 77:303–337. https://doi.org/10.1086/715398
Kirchhoff CA, Wilson ML, Mjungu DC et al (2018) Infanticide in chimpanzees: taphonomic case studies from Gombe. Am J Phys Anthropol 165:108–122. https://doi.org/10.1002/ajpa.23335
Kutsukake N, Matsusaka T (2002) Incident of intense aggression by chimpanzees against an infant from another group in Mahale Mountains National Park, Tanzania. Am J Primatol 58:175–180. https://doi.org/10.1002/ajp.10058
Lindshield S, Hernandez-Aguilar RA, Korstjens AH et al (2021) Chimpanzees (Pan troglodytes) in savanna landscapes. Evol Anthropol 30:399–420. https://doi.org/10.1002/evan.21924
Lonsdorf EV, Stanton MA, Pusey AE, Murray CM (2020) Sources of variation in weaned age among wild chimpanzees in Gombe National Park, Tanzania. Am J Phys Anthropol 171:419–429. https://doi.org/10.1002/ajpa.23986
Manson JH, Wrangham RW (1991) Intergroup aggression in chimpanzees and humans. Curr Anthropol 32:369–377. https://doi.org/10.1086/203974
Moore J (1992) “Savanna” chimpanzees. In: Nishida T, McGrew WC, Marler P, Pickford M, de Waal FBM (eds) Topics in primatology, vol 1. Human origins. Univ of Tokyo Press, Tokyo, pp 99–118
Moore J (1996) Savanna chimpanzees, referential models and the last common ancestor. In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. Cambridge Univ Press, pp 275–292
Muller MN (2017) Introduction: Chimpanzees and human evolution. In: Muller MN, Wrangham RW, Pilbeam DR (eds) Chimpanzees and human evolution. Belknap Press of Harvard Univ Press, Cambridge, MA, pp 3–21
Muller MN, Mitani JC (2005) Conflict and cooperation in wild chimpanzees. Adv Stud Behav 35:275–331. https://doi.org/10.1016/S0065-3454(05)35007-8
Newton-Fisher NE (1999) Infant killers of Budongo. Folia Primatol 70:167–169. https://doi.org/10.1159/000021690
Nishida T, Uehara S, Nyundo R (1979) Predatory behavior among wild chimpanzees of the Mahale Mountains. Primates 20:1–20. https://doi.org/10.1007/BF02373826
Piel AK, Strampelli P, Greathead E et al (2017) The diet of open-habitat chimpanzees (Pan troglodytes schweinfurthii) in the Issa valley, western Tanzania. J Hum Evol 112:57–69. https://doi.org/10.1016/j.jhevol.2017.08.016
Pruetz JD, Bertolani P (2009) Chimpanzee (Pan troglodytes verus) behavioral responses to stresses associated with living in a savanna-mosaic environment: Implications for hominin adaptations to open habitats. Paleoanthropol 2009:252–262. https://doi.org/10.4207/PA.2009.ART33
Pruetz JD, Herzog NM (2017) Savanna chimpanzees at Fongoli, Senegal, navigate a fire landscape. Curr Anthropol 58:S337–S350. https://doi.org/10.1086/692112
Pruetz JD, Ontl KB, Cleaveland E et al (2017) Intragroup lethal aggression in West African chimpanzees (Pan troglodytes verus): Inferred killing of a former alpha male at Fongoli, Senegal. Int J Primatol 38:31–57. https://doi.org/10.1007/s10764-016-9942-9
Pusey AE (2001) Of genes and apes: chimpanzee social organisation and reproduction. In: de Waal FBM (ed) Tree of origin. Harvard Univ Press, Cambridge, MA, pp 11–37
Pusey AE, Oehlert GW, Williams JM, Goodall J (2005) Influence of ecological and social factors on body mass of wild chimpanzees. Int J Primatol 26:3–3. https://doi.org/10.1007/s10764-005-0721-21
Samson DR, Hunt KD (2014) Is chimpanzee (Pan troglodytes schweinfurthii) low population density linked with low levels of aggression? Pan Afr News 21:15–17
Sherrow HM, Amsler SJ (2007) New intercommunity infanticides by the chimpanzees of Ngogo, Kibale National Park, Uganda. Int J Primatol 28:9–22. https://doi.org/10.1007/s10764-006-9112-6
Suzuki A (1971) Carnivory and cannibalism observed among forest-living chimpanzees. J Anthrop Soc Nippon 79:30–48. https://doi.org/10.1537/ase1911.79.30
Tutin CEG, Fernandez M (1991) Responses of wild chimpanzees and gorillas to the arrival of primatologists: behavior observed during habituation. In: Box HO (ed) Primate responses to environmental change. Springer, Dordrecht, pp 187–197. https://doi.org/10.1007/978-94-011-3110-0_10
van Leeuwen KL, Hill RA, Korstjens AH (2020) Classifying chimpanzee (Pan troglodytes) landscapes across large-scale environmental gradients in Africa. Int J Primatol 41:800–821. https://doi.org/10.1007/s10764-020-00164-5
Watts DP, Mitani JC (2000) Infanticide and cannibalism by male chimpanzees at Ngogo, Kibale National Park, Uganda. Primates 41:357–365. https://doi.org/10.1007/BF02557646
Watts DP, Mitani JC (2001) Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour 138:299–327
Watts DP, Mitan JC, Sherrow HM (2002) New cases of inter-community infanticide by male chimpanzees at Ngogo, Kibale National Park, Uganda. Primates 43:263–270. https://doi.org/10.1007/BF02629601
Watts DP, Muller M, Amsler SJ et al (2006) Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am J Primatol 68:161–180. https://doi.org/10.1002/ajp.20214
Williams JM, Oehlert GW, Jv C, Pusey AE (2004) Why do male chimpanzees defend a group range? Anim Behav 68:523–532. https://doi.org/10.1016/j.anbehav.2003.09.015
Wilson ML, Glowacki L (2017) Violent cousins: chimpanzees, humans, and the roots of war. In: Muller MN, Wrangham RW, Pilbeam DR (eds) Chimpanzees and human evolution. Belknap Press of Harvard Univ Press, Cambridge, MA, pp 464–508
Wilson ML, Wrangham RW (2003) Intergroup relations in Chimpanzees. Annu Rev Anthropol 32:363–392. https://doi.org/10.1146/annurev.anthro.32.061002.120046
Wilson ML, Wallauer WR, Pusey AE (2004) New cases of intergroup violence among chimpanzees in Gombe National Park, Tanzania. Int J Primatol 18:523–549. https://doi.org/10.1023/B:IJOP.0000023574.38219.92
Wilson ML, Kahlenberg SM, Wells M, Wrangham RW (2012) Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim Behav 83:277–291. https://doi.org/10.1016/j.anbehav.2011.11.004
Wilson ML, Boesch C, Fruth B et al (2014) Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513:414–417. https://doi.org/10.1038/nature13727
Wilson ML, Cossette J, Koops K et al (2015) The most unkindest cut: genital wounding by chimpanzees. Am J Phys Anthropol 156:326
Wrangham RW (1979) On the evolution of ape social systems. Soc Sci Inf 18:336–368. https://doi.org/10.1177/053901847901800301
Wrangham RW (1999) Evolution of coalitionary killing. Am J Phys Anthropol 110:1–30. https://doi.org/10.1002/(SIC)1096-8644(1999)110:29+%3c1::AID-AJPA%3e3.0.CO;2-E
Wrangham RW, Benenson J (2017) Cooperative and competitive relationships within sexes. In: Muller MN, Wrangham RW, Pilbeam DR (eds) Chimpanzees and human evolution. Belknap Press of Harvard Univ Press, Cambridge, MA, pp 509–547
Wrangham RW, Wilson ML, Muller MN (2006) Comparative rates of violence in chimpanzees and humans. Primates 47:14–26. https://doi.org/10.1007/s10329-005-0140-1
Wrangham RW, Smuts BB (1980) Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J Reprod Fertil Supplement 13–31
Acknowledgements
We thank the Tanzanian Wildlife Research Institute (TAWIRI), the Commission for Science and Technology (COSTECH), and the Mpanda District government for granting permission for research to be conducted. We also thank the Greater Mahale Ecosystem Research and Conservation (GMERC) field assistants who provide crucial support and data collection. Finally, many thanks to the UCSD/Salk Center for Academic Research and Training in Anthropogeny (CARTA) and Department of Human Origins, Max Plank Institute for Evolutionary Anthropology for support of GMERC.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by RD-C, and data handling and analysis was carried out by RD-C and CF. The first draft of the manuscript was written by RD-C, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Funding was contributed by AP, FS and RD-C.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. This work was supported by UCSD/Salk Center for Academic Research and Training in Anthropogeny (CARTA).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Drummond-Clarke, R.C., Fryns, C., Stewart, F.A. et al. A case of intercommunity lethal aggression by chimpanzees in an open and dry landscape, Issa Valley, western Tanzania. Primates 64, 599–608 (2023). https://doi.org/10.1007/s10329-023-01085-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-023-01085-6