Abstract
Purpose
Glimepiride, an anti-diabetic and third-generation sulfonylurea drug belonging to class II BCS (Biopharmaceutical Classification System) type, is characterized by its low solubility and high permeability. In order to increase glimepiride’s aqueous solubility and hence increase its dissolution rate, the goal of this study was to formulate the drug as binary and ternary solid dispersion employing water-soluble carriers.
Methods
Three binary solid dispersions of glimepiride were prepared by solvent evaporation technique using β-cyclodextrin with different drug carrier ratios. After optimizing the binary solid dispersion concerning solubility improvement, four different ratios of ternary solid dispersion employing polyvinylpyrrolidone K30 (PVPK30) were fabricated with the optimized solid dispersion to determine solubility and compared with marketed formulation to determine the consequence of the study. Further FTIR, XRD, and DSC studies were performed for a better understanding of the characterization of optimized solid dispersion and to know if there are any significant interactions with water-soluble carriers or with excipients.
Results
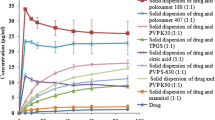
The combination of the glimepiride and β-cyclodextrin systems significantly increases the solubility and in the case of ternary solid dispersion, the solubility is increased even more. The enhancement of the solubility is influenced by the carrier’s concentration.
Conclusions
A total of four tablet formulation batches that were prepared and the explicit in vitro comparisons been carried out with commercially available immediate-release formulation, suggested that formulating glimepiride in ternary solid dispersion has enhanced the solubility and dissolution rate drastically.
Graphical Abstract





Similar content being viewed by others
Data Availability
Data are contained within the article.
Abbreviations
- T2DM:
-
Type II diabetes mellitus
- BCS:
-
Biopharmaceutical classification system
- BCD:
-
β-Cyclodextrin
- PVPK30:
-
Polyvinylpyrrolidone K30
- MCC:
-
Microcrystalline cellulose
- SD:
-
Solid dispersion
- PM:
-
Physical mixture
- GLIMI:
-
Glimepiride
- FTIR:
-
Fourier-transform infrared spectroscopy
- MW:
-
Molecular weight
- XRD:
-
X-ray diffraction
- DSC:
-
Differential scanning calorimetry
References
Basit A, Riaz M, Fawwad A. Glimepiride: evidence-based facts, trends, and observations. Vasc Health Risk Manag. 2012;463–472.
Akhter S, Hossen MS, Salahuddin M, Sunny MA, Sathi FA, Islam MS. In vitro dissolution study of glimepiride from binary and ternary solid dispersion formulation. Univers J Pharm Res. 2019;4(5):7–12.
Malkawi R, Malkawi WI, Al-Mahmoud Y, Tawalbeh J. Current trends on solid dispersions: past, present, and future. Adv Pharmacol Pharm Sci. 2022;2022.
Gurunath S, Kumar SP, Basavaraj NK, Patil PA. Amorphous solid dispersion method for improving oral bioavailability of poorly water-soluble drugs. J Pharm Res. 2013;6(4):476–80.
Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2(1):12.
Nikam VK, Shete SK, Khapare JP. Most promising solid dispersion technique of oral dispersible tablet. Beni-Suef Univ J Basic Appl Sci. 2020;9:1–16.
Qushawy M, Nasr A, Swidan S, Mortagi Y. Development and characterization of glimepiride novel solid nanodispersion for improving its oral bioavailability. Sci Pharm. 2020;88(4):52.
Yuvaraja K, Khanam J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J Pharm Biomed Anal. 2014;96:10–20.
Das SK, Kahali N, Bose A, Khanam J. Physicochemical characterization and in vitro dissolution performance of ibuprofen-Captisol®(sulfobutylether sodium salt of β-CD) inclusion complexes. J Mol Liq. 2018;261:239–49.
Maulvi FA, Dalwadi SJ, Thakkar VT, Soni TG, Gohel MC, Gandhi TR. Improvement of dissolution rate of aceclofenac by solid dispersion technique. Powder Technol. 2011;207(1–3):47–54.
Mehta A, Vasanti S, Tyagi R, Shukla A. Formulation and evaluation of solid dispersions of an anti-diabetic drug. Curr Trends Biotechnol Pharm. 2009;3(1):76–84.
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49.
Adhikari L, Semalty M, Naruka PS, Aswal VK, Semalty A. Binary complexes of glimepiride with β-cyclodextrin for improved solubility and drug delivery. Indian Drugs. 2019;56(3):54–60.
Tran P, Pyo YC, Kim DH, Lee SE, Kim JK, Park JS. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics. 2019;11(3):132.
Daravath B, Naveen C, Vemula SK, Tadikonda RR. Solubility and dissolution enhancement of flurbiprofen by solid dispersion using hydrophilic carriers. Braz J Pharm Sci 2018;53.
Choi JS, Lee SE, Jang WS, Byeon JC, Park JS. Solid dispersion of dutasteride using the solvent evaporation method: approaches to improve dissolution rate and oral bioavailability in rats. Mater Sci Eng, C. 2018;90:387–96.
Hanada M, Jermain SV, Thompson SA, Furuta H, Fukuda M, Williams RO III. Ternary amorphous solid dispersions containing a high-viscosity polymer and mesoporous silica enhance dissolution performance. Mol Pharm. 2020;18(1):198–213.
Nazmi M, Islam SA, Bhuiyan MA, Reza MS. Effect of superdisintegrants and their mode of incorporation on disintegration time and release profile of carbamazepine from immediate release tablet. J Appl Pharm Sci. 2013;3(5):080–4.
Kishore K, Sudhakara RP, Srininvas RD, Maneshwar T, Kiran KV, Raju L. Preparation and characterization of oro dispersible tablets of glimepride-pvp k30 solid dispersion. Int J Biol Pharm Res. 2013;4(8):547–55.
Nagpal M, Rajera R, Nagpal K, Rakha P, Singh SK, Mishra DN. Dissolution enhancement of glimepiride using modified gum karaya as a carrier. Int J Pharm Investig. 2012;2(1):42.
Gill B, Kaur T, Kumar S, Gupta GD. Formulation and evaluation of glimepiride solid dispersion tablets. Asian J Pharm (AJP). 2010;4(3).
Ibrahim AH, Rosqvist E, Smått JH, Ibrahim HM, Ismael HR, Afouna MI, Samy AM, Rosenholm JM. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int J Pharm. 2019;563:217–27.
Silberberg M. Cyclodextrin as a drug carrier increasing drug solubility. The Science Journal of the Lander College of Arts and Sciences. 2017;11(1):5.
Seitz JA, Flessland GM. Evaluation of the physical properties of compressed tablets I: tablet hardness and friability. J Pharm Sci. 1965;54(9):1353–7.
Siddiqui MN, Garg G, Sharma PK. Fast dissolving tablets: preparation, characterization and evaluation: an overview. Int J Pharm Sci Rev Res. 2010;4(2):87–96.
Sekar V, Chellan VR. Immediate release tablets of telmisartan using superdisintegrant-formulation, evaluation and stability studies. Chem Pharm Bull. 2008;56(4):575–7.
Grbic S, Parojcic J, Ibric S, Djuric Z. In vitro–in vivo correlation for gliclazide immediate-release tablets based on mechanistic absorption simulation. AAPS PharmSciTech. 2011;12:165–71.
Medina JR, Salazar DK, Hurtado M, Cortes AR, Domínguez-Ramírez AM. Comparative in vitro dissolution study of carbamazepine immediate-release products using the USP paddles method and the flow-through cell system. Saudi Pharm J. 2014;22(2):141–7.
Reddy NH, Patnala S, Löbenberg R, Kanfer I. In vitro dissolution of generic immediate-release solid oral dosage forms containing BCS class I drugs: comparative assessment of metronidazole, zidovudine, and amoxicillin versus relevant comparator pharmaceutical products in South Africa and India. AAPS PharmSciTech. 2014;15:1076–86.
Acknowledgements
The authors appreciate the gift sample of glimepiride from Dr Reddy’s Laboratories Ltd. (India) and the department of Pharmaceutical Technology and department of Metallurgical and Material engineering at Jadavpur University (Kolkata-700032, India) for providing all necessary facilities. The authors are also thankful of the AICTE’s financial assistance for their work.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.C., N.A., and K.K.; methodology: R.C.; software: N.A.; validation: R.C., N.A., and K.K.; formal analysis: R.C. and N.A.; investigation: R.C.; resources: K.K.; data curation: R.C. and N.A.; visualization: R.C. and N.A.; writing—original draft preparation and review and editing: R.C.; visualization: R.C. and N.A.; supervision: K.K.; project administration: R.C., N.A., and K.K.
Corresponding author
Ethics declarations
Ethics Approval
N/A.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakraborty, R., Afrose, N. & Kuotsu, K. A Potential Breakthrough in the Enhancement of Glimepiride Solubility and Dissolution Rate by Binary and Ternary Solid Dispersion Technique and In Vitro Comparison with Marketed Formulation. J Pharm Innov 18, 1981–1991 (2023). https://doi.org/10.1007/s12247-023-09761-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09761-2