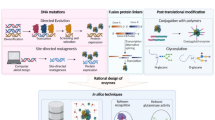
Abstract
Introduction
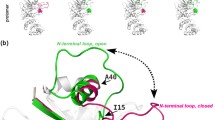
L-asparaginase (also known as L-ASNase) is a crucial therapeutic enzyme that is widely used in treatment of ALL (acute lymphoblastic leukemia) as a chemotherapeutic drug. Besides, this enzyme is used in the food industry as a food processing reagent to reduce the content of acrylamide in addition to the clinical industry. The improvement of activity and kinetic parameters of the L-ASNase enzyme may lead to higher efficiency resulting in practical achievement. In order to achieve this goal, we chosen glycine residue in position 88 as a potential mutation with advantageous outcomes.
Method
In this study, firstly to find the appropriate mutation on glycine 88, various in silico analyses, such as MD simulation and molecular docking, were carried out. Then, the rational design was adopted as the best strategy for molecular modifications of the enzyme to improve its enzymatic properties.
Result
Our in silico findings show that the four mutations G88Q, G88L, G88K, and G88A may be able to increase L-ASNase’s asparaginase activity. The catalytic efficiency of each enzyme (kcat/Km) is the most important feature for comparing the catalytic activity of the mutants with the wild type form. The laboratory experiments showed that the kcat/Km for the G88Q mutant is 36.32% higher than the Escherichia coli K12 ASNase II (wild type), which suggests that L-ASNase activity is improved at lower concentration of L-ASN. Kinetic characterization of the mutants L-ASNase activity confirmed the high turnover rate (kcat) with ASN as substrate relative to the wild type enzyme.
Conclusion
In silico analyses and laboratory experiments demonstrated that the G88Q mutation rather than other mutation (G88L, G88K, and G88A) could improve the kinetics of L-ASNase.




Similar content being viewed by others
Data Availability
Not applicable.
References
Maggi M, Scotti C (2022) Structural aspects of E. coli type II asparaginase in Complex with its secondary product L-Glutamate. Int J Mol Sci 23(11):5942
Wang Y, Xu W, Wu H, Zhang W, Guang C, Mu W (2021) Microbial production, molecular modification, and practical application of L-asparaginase: a review. Int J Biol Macromol 186:975–983
Van Trimpont M, Peeters E, De Visser Y, Schalk AM, Mondelaers V, De Moerloose B et al (2022) Novel insights on the Use of L-Asparaginase as an efficient and safe anti-cancer therapy. Cancers 14(4):902
Wang N, Ji W, Wang L, Wu W, Zhang W, Wu Q et al (2022) Overview of the structure, side effects, and activity assays of l-asparaginase as a therapy drug of acute lymphoblastic leukemia. RSC Med Chem.
Avramis VI, Tiwari PN (2006) Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int J Nanomed 1(3):241
Mohan Kumar N, Shimray CA, Indrani D, Manonmani H (2014) Reduction of acrylamide formation in sweet bread with L-asparaginase treatment. Food Bioprocess Technol 7(3):741–748
Kukurová K, Morales FJ, Bednarikova A, Ciesarova Z (2009) Effect of l-asparaginase on acrylamide mitigation in a fried‐dough pastry model. Mol Nutr Food Res 53(12):1532–1539
Huang L, Liu Y, Sun Y, Yan Q, Jiang Z (2014) Biochemical characterization of a novel L-Asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl Environ Microbiol 80(5):1561–1569
Mahboobi M, Sedighian H, Hedayati C, Bambai B, Soofian SE, Amani J (2017) Applying bioinformatic tools for modeling and modifying type II E. coli L-Asparginase to present a better therapeutic agent/drug for acute lymphoblastic leukemia. Iran J Cancer Prev 10(3):10
Keshtvarz M, Mahboobi M, Kieliszek M, Miecznikowski A, Sedighian H, Rezaei M et al (2021) Engineering of cytolethal distending toxin B by its reducing immunogenicity and maintaining stability as a new drug candidate for tumor therapy; an in silico study. Toxins 13(11):785
Galande S, Khaursade P, Prakasham RS, Kishor PK, IN-SILICO DEVELOPMENT, OF EFFICIENT L-ASPARAGINASE ENZYME FOR ACUTE LYMPHOBLASTIC LEUKAEMIA THERAPY. INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH (2018). ;9(10):4177–4186
Xia Y, Chu W, Qi Q, Xun L (2015) New insights into the QuikChangeTM process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res 43(2):e12–e
Xia Y, Xun L (2017) Revised mechanism and improved efficiency of the quikchange site-directed mutagenesis method. In Vitro Mutagenesis. Springer; p. 367 – 74
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:172
Sambrook J (2001) A laboratory manual. Molecular cloning. ;1
Froimowitz M (1993) HyperChem: a software package for computational chemistry and molecular modeling. Biotechniques 14(6):1010–1013
Swain AL, Jaskólski M, Housset D, Rao J, Wlodawer A (1993) Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Proceedings of the National Academy of Sciences. ;90(4):1474-8
Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI et al (2021) UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 30(1):70–82
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng Des selection 8(2):127–134
Eswar N, Webb B, Marti-Renom MA, Madhusudhan M, Eramian D, Shen My et al (2006) Comparative protein structure modeling using Modeller. Curr protocols Bioinf 15(1):561–5630
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins Struct Funct Bioinform 65(3):712–725
Oostenbrink C, Villa A, Mark AE, Van Gunsteren WF (2004) A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J Comput Chem 25(13):1656–1676
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N⋅ log (N) method for Ewald sums in large systems. J Chem Phys 98(12):10089–10092
Hess B, Bekker H, Berendsen HJ, Fraaije JG (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18(12):1463–1472
An Y, Chen L, Sun S, Lv A, Wu W (2011) QuikChange shuffling: a convenient and robust method for site-directed mutagenesis and random recombination of homologous genes. New Biotechnol 28(4):320–325
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10(5):411–421
Naderi M, Ghaderi R, Khezri J, Bambai AKB (2022) Crucial role of non-hydrophobic residues in H-region signal peptide on secretory production of l-asparaginase II in Escherichia coli. Biochemical and Biophysical Research Communications
Li L, Park E, Ling J, Ingram J, Ploegh H, Rapoport TA (2016) Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature 531(7594):395–399
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Shifrin S, Parrott CL, Luborsky SW (1974) Substrate binding and intersubunit interactions in L-asparaginase. J Biol Chem 249(5):1335–1340
Aghaeepoor M, Akbarzadeh A, Mirzaie S, Hadian A, Aval SJ, Dehnavi E (2018) Selective reduction in glutaminase activity of l–Asparaginase by asparagine 248 to serine mutation: a combined computational and experimental effort in blood cancer treatment. Int J Biol Macromol 120:2448–2457
Vimal A, Kumar A (2017) Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol Genet Eng Rev 33(1):40–61
Mohan Kumar N, Manonmani H (2013) Purification, characterization and kinetic properties of extracellular L-asparaginase produced by Cladosporium sp. World J Microbiol Biotechnol 29(4):577–587
Lu X, Chen J, Jiao L, Zhong L, Lu Z, Zhang C et al (2019) Improvement of the activity of L-asparaginase I improvement of the catalytic activity of L-asparaginase I from Bacillus megaterium H-1 by in vitro directed evolution. J Biosci Bioeng 128(6):683–689
Parvin S, Sedighian H, Sohrabi E, Mahboobi M, Rezaei M, Ghasemi D et al (2022) Prediction of genes involved in lung cancer with a systems biology approach based on comprehensive gene information. Biochem Genet 60(4):1253–1273
Shabgah AG, Navashenaq JG, Mahboobi M, Sedighian H (2014) Immunotherapy as an optimal manner in cancer treatment. Biosci Biotechnol Res Asia 11(3):1167–1178
Harms E, Wehner A, Aung H-P, Röhm K (1991) A catalytic role for threonine-12 of E. coli asparaginase II as established by site‐directed mutagenesis. FEBS Lett 285(1):55–58
Derst C, Henseling J, Röhm K (1992) Probing the role of threonine and serine residues of E. coli asparaginase II by site-specific mutagenesis. Protein Eng Des Selection 5(8):785–789
Derst C, Henseling J, Röhm K-H (2000) Engineering the substrate specificity of Escherichia coli asparaginase II. Selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Sci 9(10):2009–2017
Mehta RK, Verma S, Pati R, Sengupta M, Khatua B, Jena RK et al (2014) Mutations in subunit interface and B-cell epitopes improve antileukemic activities of Escherichia coli asparaginase-II: evaluation of immunogenicity in mice. J Biol Chem 289(6):3555–3570
Verma S, Mehta RK, Maiti P, Röhm K-H, Sonawane A (2014) Improvement of stability and enzymatic activity by site-directed mutagenesis of E. coli asparaginase II. Biochimica et Biophysica Acta (BBA)-Proteins and proteomics. ;1844(7):1219–1230
Belén LH, Lissabet JB, de Oliveira Rangel-Yagui C, Effer B, Monteiro G, Pessoa A et al (2019) A structural in silico analysis of the immunogenicity of l-asparaginase from Escherichia coli and Erwinia carotovora. Biologicals 59:47–55
Kleiner-Grote GR, Risse JM, Friehs K (2018) Secretion of recombinant proteins from E. coli. Eng Life Sci 18(8):532–550
Yoon SH, Kim SK, Kim JF (2010) Secretory production of recombinant proteins in Escherichia coli. Recent Patents Biotechnol 4(1):23–29
Owji H, Nezafat N, Negahdaripour M, Hajiebrahimi A, Ghasemi Y (2018) A comprehensive review of signal peptides: structure, roles, and applications. Eur J Cell Biol 97(6):422–441
Erkut E (2021) Bacterial signal peptides: structure, optimization, and applications. Eureka. ;6(1)
Tsirigotaki A, De Geyter J, Economou A, Karamanou S (2017) Protein export through the bacterial sec pathway. Nat Rev Microbiol 15(1):21–36
Freudl R (2018) Signal peptides for recombinant protein secretion in bacterial expression systems. Microb Cell Fact 17(1):1–10
Nguyen HA, Su Y, Lavie A (2016) Design and characterization of erwinia chrysanthemi l-asparaginase variants with diminished l-glutaminase activity. J Biol Chem 291(34):17664–17676
Acknowledgements
We thank our colleagues from “NIGEB and Applied Microbiology Research Center” who provided insight and expertise that greatly assisted our research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MM Conceptualization, Methodology, Validation, formal analysis, Investigation, wrote the manuscript draft, Visualization. AHS and BB, Conceptualization, Writing review & editing and supervision. MM and HS revized the manuscript and bioinformatic analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahboobi, M., Salmanian, AH., Sedighian, H. et al. Molecular Modeling and Optimization of Type II E.coli l-Asparginase Activity by in silico Design and in vitro Site-directed Mutagenesis. Protein J 42, 664–674 (2023). https://doi.org/10.1007/s10930-023-10149-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-023-10149-x