Abstract
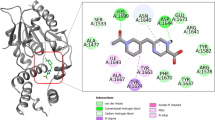
Multidrug-resistant tuberculosis (MDR-TB) continues to spread worldwide and remains one of the leading causes of death among infectious diseases. The enoyl-acyl carrier protein reductase (InhA) belongs to FAS-II family and is essential for the formation of the Mycobacterium tuberculosis cell wall. Recent years, InhA direct inhibitors have been extensively studied to overcome MDR-TB. However, there are still no inhibitors that have entered clinical research. Here, the ensemble docking-based virtual screening along with biological assay were used to identify potent InhA direct inhibitors from Chembridge, Chemdiv, and Specs. Ultimately, 34 compounds were purchased and first assayed for the binding affinity, of which four compounds can bind InhA well with KD values ranging from 48.4 to 56.2 µM. Among them, compound 9,222,034 has the best inhibitory activity against InhA enzyme with an IC50 value of 18.05 µM. In addition, the molecular dynamic simulation and binding free energy calculation indicate that the identified compounds bind to InhA with “extended” conformation. Residue energy decomposition shows that residues such as Tyr158, Met161, and Met191 have higher energy contributions in the binding of compounds. By analyzing the binding modes, we found that these compounds can bind to a hydrophobic sub-pocket formed by residues Tyr158, Phe149, Ile215, Leu218, etc., resulting in extensive van der Waals interactions. In summary, this study proposed an efficient strategy for discovering InhA direct inhibitors through ensemble docking-based virtual screening, and finally identified four active compounds with new skeletons, which can provide valuable information for the discovery and optimization of InhA direct inhibitors.







Similar content being viewed by others
References
Ou ZJ et al (2021) Trends in burden of multidrug-resistant tuberculosis in countries, regions, and worldwide from 1990 to 2017: results from the Global Burden of Disease study. Infect Dis Poverty 10(1):24
Kaul G et al (2019) Management of multidrug-resistant tuberculosis in the 21st century. Drugs Today (Barc) 55(3):215–224
World Health Organization. Global tuberculosis report 2021
Wilson JW, Nilsen DM, Marks SM (2020) Multidrug-resistant tuberculosis in patients with human immunodeficiency virus. Management considerations within high-resourced settings. Ann Am Thorac Soc 17(1):16–23
Singh P et al (2018) Cell envelope lipids in the pathophysiology of Mycobacterium tuberculosis. Future Microbiol 13:689–710
Bhatt A, Besra MV, Jacobs GS, Kremer WR Jr (2007) The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol Microbiol 64(6):1442–1454
Duan X, Xiang X, Xie J (2014) Crucial components of mycobacterium type II fatty acid biosynthesis (Fas-II) and their inhibitors. FEMS Microbiol Lett 360(2):87–99
Gurvitz A, Hiltunen JK, Kastaniotis AJ (2008) Function of heterologous Mycobacterium tuberculosis InhA, a type 2 fatty acid synthase enzyme involved in extending C20 fatty acids to C60-to-C90 mycolic acids, during de novo lipoic acid synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol 74(16):5078–5085
Marrakchi H, Lanéelle G, Quémard AK (2000) InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Microbiology 146(Pt 2):289–296
Banerjee A et al (1994) inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263(5144):227–230
Vilcheze C et al (2006) Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat Med 12(9):1027–1029
Stigliani JL et al (2008) Binding of the tautomeric forms of isoniazid-NAD adducts to the active site of the Mycobacterium tuberculosis enoyl-ACP reductase (InhA): a theoretical approach. J Mol Graph Model 27(4):536–545
Ahmad S et al (2002) Prevalence of S315T mutation within the katG gene in isoniazid-resistant clinical Mycobacterium tuberculosis isolates from Dubai and Beirut. Int J Tuberc Lung Dis 6(10):920–926
Hoagland DT et al (2016) New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv Drug Deliv Rev 102:55–72
Martínez-Hoyos M et al (2016) Antitubercular drugs for an old target: GSK693 as a promising InhA direct inhibitor. EBioMedicine 8:291–301
Chollet A et al (2018) An overview on crystal structures of InhA protein: Apo-form, in complex with its natural ligands and inhibitors. Eur J Med Chem 146:318–343
Chhibber M et al (2006) Novel diphenyl ethers: design, docking studies, synthesis and inhibition of enoyl ACP reductase of Plasmodium falciparum and Escherichia coli. Bioorg Med Chem 14(23):8086–8098
Chetty S et al (2021) New InhA inhibitors based on expanded Triclosan and Di-Triclosan Analogues to develop a New Treatment for Tuberculosis. Pharmaceuticals (Basel), 14(4)
Freundlich JS et al (2009) Triclosan derivatives: towards potent inhibitors of drug-sensitive and drug-resistant Mycobacterium tuberculosis. ChemMedChem 4(2):241–248
He X et al (2006) Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis. J Med Chem 49(21):6308–6323
He X, Alian A, Ortiz de PR, Montellano (2007) Inhibition of the Mycobacterium tuberculosis enoyl acyl carrier protein reductase InhA by arylamides. Bioorg Med Chem 15(21):6649–6658
Guardia A et al (2016) Benzyl-4-((heteroaryl)methyl)benzamides: a New Class of Direct NADH-Dependent 2-trans enoyl-acyl carrier protein reductase (InhA) inhibitors with antitubercular activity. ChemMedChem 11(7):687–701
Kuo MR et al (2003) Targeting tuberculosis and malaria through inhibition of Enoyl reductase: compound activity and structural data. J Biol Chem 278(23):20851–20859
Encinas L et al (2014) Encoded library technology as a source of hits for the discovery and lead optimization of a potent and selective class of bactericidal direct inhibitors of Mycobacterium tuberculosis InhA. J Med Chem 57(4):1276–1288
Soutter HH et al (2016) Discovery of cofactor-specific, bactericidal Mycobacterium tuberculosis InhA inhibitors using DNA-encoded library technology. Proc Natl Acad Sci U S A 113(49):E7880–E7889
Sabbah M et al (2020) Fragment-based design of Mycobacterium tuberculosis InhA inhibitors. J Med Chem 63(9):4749–4761
Singh K et al (2022) Identification of novel inhibitor of Enoyl-Acyl carrier protein reductase (InhA) enzyme in Mycobacterium tuberculosis from Plant-Derived Metabolites: an in Silico Study. Antibiot (Basel), 11(8)
Kamsri P et al (2020) Discovery of New and potent InhA inhibitors as Antituberculosis Agents: structure-based virtual screening validated by biological assays and X-ray crystallography. J Chem Inf Model 60(1):226–234
Flint L, Korkegian A, Parish T (2020) InhA inhibitors have activity against non-replicating Mycobacterium tuberculosis. PLoS ONE 15(11):e0239354
Luckner SR et al (2010) A slow, tight binding inhibitor of InhA, the enoyl-acyl carrier protein reductase from Mycobacterium tuberculosis. J Biol Chem 285(19):14330–14337
Holas O, Ondrejcek P, Dolezal M (2015) Mycobacterium tuberculosis enoyl-acyl carrier protein reductase inhibitors as potential antituberculotics: development in the past decade. J Enzyme Inhib Med Chem 30(4):629–648
López-Vallejo F et al (2011) Integrating virtual screening and combinatorial chemistry for accelerated drug discovery. Comb Chem High Throughput Screen 14(6):475–487
Slater O, Kontoyianni M (2019) The compromise of virtual screening and its impact on drug discovery. Expert Opin Drug Discov 14(7):619–637
da Silva Rocha SFL et al (2019) Virtual screening techniques in Drug Discovery: review and recent applications. Curr Top Med Chem 19(19):1751–1767
Cleves AE, Jain AN (2020) Structure- and ligand-based virtual screening on DUD-E(+): performance dependence on Approximations to the binding Pocket. J Chem Inf Model 60(9):4296–4310
Olaru A et al (2015) Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit Rev Anal Chem 45(2):97–105
Mysinger MM et al (2012) Directory of useful decoys, enhanced (DUD-E): better ligands and decoys for better benchmarking. J Med Chem 55(14):6582–6594
Huang N, Shoichet BK, Irwin JJ (2006) Benchmarking sets for molecular docking. J Med Chem 49(23):6789–6801
Case DAB, Ben-Shalom K, Brozell IY, Cerutti SR, Cheatham DS, Cruzeiro TEIII, Darden VWD, Duke TA, Giambasu RE (2020) G.; et al., AMBER 2020. University of California, San Francisco, CA, USA
Genheden S, Ryde U (2012) Comparison of end-point continuum‐solvation methods for the calculation of protein–ligand binding free energies Proteins: Structure, Function, and Bioinformatics, 80(5): p. 1326–1342
Wang E et al (2019) End-point binding Free Energy calculation with MM/PBSA and MM/GBSA: strategies and applications in Drug Design. Chem Rev 119(16):9478–9508
Tan S et al (2022) Molecular modeling study on the Interaction mechanism between the LRRK2 G2019S mutant and type I inhibitors by integrating Molecular Dynamics Simulation, binding Free Energy Calculations, and Pharmacophore modeling. ACS Chem Neurosci 13(5):599–612
Zhang Q et al (2022) Binding thermodynamics and dissociation Kinetics Analysis uncover the key structural motifs of phenoxyphenol derivatives as the direct InhA inhibitors and the Hotspot residues of InhA. Int J Mol Sci 23(17):10102
Phusi N et al (2023) Structure-based drug design of novel M. tuberculosis InhA inhibitors based on fragment molecular orbital calculations. Comput Biol Med 152:106434
Shirude PS et al (2013) Methyl-thiazoles: a novel mode of inhibition with the potential to develop novel inhibitors targeting InhA in Mycobacterium tuberculosis. J Med Chem 56(21):8533–8542
Rozwarski DA et al (1999) Crystal structure of the Mycobacterium tuberculosis enoyl-ACP reductase, InhA, in complex with NAD + and a C16 fatty acyl substrate. J Biol Chem 274(22):15582–15589
Lipinski CA et al (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46(1–3):3–26
Halgren TA et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47(7):1750–1759
Friesner RA et al (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49(21):6177–6196
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10(5):449–461
Parikh SL, Xiao G, Tonge PJ (2000) Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 39(26):7645–7650
Frisch MT, Schlegel G, Scuseria H, Robb G, Cheeseman M, Scalmani J, Barone G, Petersson V, Nakatsuji G (2016) H., Gaussian 16. Gaussian. Inc., Wallingford, CT, USA
Kristyán S, Ruzsinszky A, Csonka GI (2001) Accurate thermochemistry from corrected hartree–fock results: rapid estimation of nearly experimental quality total energy using the small 6-31G (d) basis set. Theor Chem Acc 106:319–328
Tian C et al (2019) ff19SB: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J Chem Theory Comput 16(1):528–552
Wang J et al (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174
Price DJ, Brooks CL (2004) A modified TIP3P water potential for simulation with Ewald summation. J Chem Phys 121(20):10096–10103
Joung IS, Cheatham TE (2008) Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B 112(30):9020–9041
Toukmaji A et al (2000) Efficient particle-mesh Ewald based approach to fixed and induced dipolar interactions. J Chem Phys 113(24):10913–10927
Miyamoto S, Kollman PA (1992) Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem 13(8):952–962
Gilson MK et al (1993) Computation of electrostatic forces on solvated molecules using the Poisson-Boltzmann equation. J Phys Chem 97(14):3591–3600
Sitkoff D, Sharp KA, Honig B (1994) Accurate calculation of hydration free energies using macroscopic solvent models. J Phys Chem 98(7):1978–1988
Weiser J, Shenkin PS, Still WC (1999) Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J Comput Chem 20(2):217–230
Pearlman DA et al (1995) AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput Phys Commun 91(1–3):1–41
Acknowledgements
This work was supported by Macao Polytechnic University (No. RP/FCA-01/2022).
Author information
Authors and Affiliations
Contributions
Huanxiang Liu and Qianqian Zhang wrote the main manuscript text and Qianqian Zhang, Jianting Han, Yongchang Zhu, Fansen Yu, Xiaopeng Hu, Henry H. Y. Tong performed the virtual screening, biological assays and prepared all figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Han, J., Zhu, Y. et al. Discovery of novel and potent InhA direct inhibitors by ensemble docking-based virtual screening and biological assays. J Comput Aided Mol Des 37, 695–706 (2023). https://doi.org/10.1007/s10822-023-00530-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-023-00530-4