Abstract
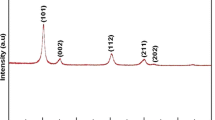
Using the sol–gel method, by reacting solutions of maleic acid and potassium permanganate, samples have been obtained with a turbostat stacking of layers, which is characteristic of the birnessite structure. Using X-ray phase and thermogravimetric analysis, Raman spectroscopy, and low-temperature nitrogen adsorption, the dependence of the textural–structural characteristics of the synthesized materials on the temperature of thermal modification (150, 360°C) and sample composition (Sr-form, H-form) has been shown. It has been found that all samples are characterized by a mesoporous texture. It is shown that the water content in the samples is closely related to the quantitative determination of the specific surface area: the more water, the greater the SBET value.




Similar content being viewed by others
REFERENCES
S. Gregg and K. Sing, Adsorption, Surface Area and Porosity (Academic Press, London, 1982; Mir, Moscow, 1984).
J. B. Miller and E. I. Ko, “Control of mixed oxide textural and acidic properties by the sol-gel method,” Catal. Today 35, 269–292 (1997).
S. Ching and J. L. Roark, “Sol-gel route to the tunneled manganese oxide cryptomelane,” Chem. Mater. 9, 750–754 (1997).
S. Franger, S. Bach, J. Farcy, and J. P. Pereira-Ramos, “Synthesis, structural and electrochemical characterizations of the sol-gel birnessite MnO1.84·0.6H2O,” Chem. Mater. 109, 262–275 (2002).
S. Ching, J. A. Londrigam, and M. J. Jorgensen, “Sol-gel synthesis of birnessite from KMnO4 and simple sugars source,” Chem. Mater. 7, 1604–1606 (1995).
Q. Zhang and S. Suib, “Transformation of cryptomalane-type manganese oxides to oxygen deficient systems by microwave-induced oxygen evolution,” Chem. Mater. 11, 1306–1311 (1999).
V. A. Drits, B. Lanson, and A.-C. Gaillot, “Birnessite polytype systematics and identifi cation by powder X‑ray diffraction,” Am. Mineral. 92, 771–788 (2007).
J. E. Post, “Manganese oxide minerals: crystal structures and economic and environmental significance,” Proc. Natl. Acad. Sci. U. S. A. 96, 3447–3454 (1999).
U. Schilling and J. R. Dahn, “Fits of the gamma-MnO2 structural model to disordered manganese dioxides,” J. Appl. Crystallogr. 31, 396–406 (1998).
M. V. Ananth, S. Pethkar, and K. Dakshinamurthi, “Distortion of MnO6 octahedra and electrochemical activity of nsutite-based MnO2 polymorphous for alkaline electrolytes–An FTIR study,” J. Power Sources 75, 278–282 (1998).
S. Turner and P. R. Buseck, “Defects in nsutite and dry-cell battery efficiency,” Nature 304, 143–146 (1983).
S. Bernardini, F. Bellatreccia, A. C. Municchia, G. D. Ventura, and A. Sodo, “Raman spectra of natural manganese oxides,” J. Raman Spectrosc. 50, 873–888 (2019).
P. Gao, P. Metz, T. Hey, Y. Gong, D. Liu, D. D. Edwards, J. Y. Howe, R. Huang, and S. T. Misture, “The critical role of point defects in improving the specific capacitance of d-MnO2 nanosheets,” Nat. Comm. 8, 14559 (2017).
F. A. AL-Sagheer and M. I. Zaki, “Surface properties of sol-gel synthesized d-MnO2 as assessed by N2 sorptometry, electron microscopy, and X-ray photoelectron spectroscopy,” Colloids Surf., A 173, 193–204 (2000).
S. Bach, M. Henry, N. Baffier, and J. Livage, “Sol-gel synthesis of manganese oxides,” J. Solid State Chem. 88, 325–333 (1990).
Funding
This work was financially supported by the Russian Foundation for Basic Research, project no. 19-43-590012 and carried out using the equipment of The Core Facilities Center “Research of materials and matter” at the PFRC UB RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Sh. Galyaltdinov
Rights and permissions
About this article
Cite this article
Saenko, E.V. Sol–Gel Synthesis and Textural–Structural Characteristics of Manganese Oxide: the Effect of Thermal-Modification Temperature. Polym. Sci. Ser. D 16, 518–521 (2023). https://doi.org/10.1134/S1995421223030279
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995421223030279