Abstract
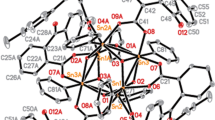
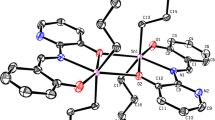
New complexes I–IV of di-tert-butyltin with ligands based on heterocyclic thioamides (2-mercaptobenzoxazole, 2-mercaptobenzothiazole, 2-mercaptobenzimidazole) and 2,6-di-tert-butyl-4-mercaptophenol are synthesized and studied by X-ray diffraction (XRD). The XRD results for single crystals of I, II, and IV are presented (CIF files CCDC nos. 2251495, 2251493, and 2251494, respectively). Specific features of the synthesized crystal structures are discussed. Complexes I and II contain the expected Sn–C and Sn–S bonds and an additional coordination with the nitrogen atom in the heterocycles, which indicates the octahedral environment of the Sn(IV) atom (coordination number 6). The coordination polyhedron in complex IV can be described as a distorted tetrahedron (coordination number 4). The proposed compounds are studied as antiproliferative agents. Their antiproliferative activity is determined using the human cancer cell lines (PC3, MCF-7, НСT116, A549, and normal cells WI38). A dependence of the activity on the ligand structure is found. A comparative evaluation of the activity shows that the introduction of the antioxidant 2,6-di-tert-butyl-4-mercaptophenol fragment into complex IV substantially decreases the cytotoxicity.



Similar content being viewed by others
REFERENCES
Milaeva, E.R., Dodokhova, M.A., Shpakovsky, D.B., et al., J. Biomed, 2021, vol. 17, p. 88. https://doi.org/10.33647/2074-5982-17-2-88-99
Antonenko, T.A., Gracheva, Yu.A., Shpakovsky, D.B., et al., Int. J. Mol. Sci., 2023, vol. 24, p. 2024.
Nikitin, E., Mironova, E., Shpakovsky, D., et al., Molecules, 2022, vol. 27, p. 8359.
Milaeva, E.R., Shpakovsky, D.B., Gracheva, Yu.A., et al., Pure Appl. Chem., 2020, vol. 92, p. 1201.
Shpakovsky, D.B., Banti, C.N., Mukhatova, E.M., et al., Dalton Trans., 2014, vol. 43, no. 18, p. 6880.
Milaeva, E.R., Shpakovsky, D.B., Gracheva, Yu.A., et al., J. Organomet. Chem., 2015, vol. 782, p. 96.
Banti, C.N., Hadjikakou, S.K., Sismanoglu, T., et al., J. Inorg. Biochem., 2019, vol. 194, p. 114.
Chaurasiya, A., Sahu, C., Wahan, S.K., et al., J. Mol. Struct., 2023, vol. 1280, p. 134967.
Bandaru, P.K., Rao, N.S., Radhika, G., et al., Chem. Data Coll., 2023, vol. 44, p. 100994.
Orafaiea, A., Mousavianb, M., Orafaic, H., et al., Prostaglandins and Other Lipid Mediators, 2020, vol. 148, p. 106411.
Xanthopoulou, M.N., Hadjikakou, S.K., Hadjiliadis, N., et al., Eur. J. Med. Chem., 2008, vol. 43, p. 327.
Muller, E., Stegman, H.B., and Scheffler, K., Liebigs Ann. Chem., 1961, vol. 645, p. 79.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., et al., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr. Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al., Wallingford (CT): Gaussian 09, Revision D.01, Gaussian, Inc., 2016.
Perdew, J., Ernzerhof, M., and Burke, K., J. Chem. Phys., 1996, vol. 105, p. 9982.
Grimme, S., Antony, J., Ehrlich, S., et al., J. Chem. Phys., 2010, vol. 132, p. 154104.
Keith, T., AIMAll (version 16.08.17), Overland Park (KS): TK Gristmill Software, 2016. https://aim.tkgristmill.com.
Mikhalev, O.V., Shpakovsky, D.B., Gracheva, Yu.A., et al., Russ. Chem. Bull., 2018, vol. 67, no. 4, p. 712. https://doi.org/10.1007/s11172-018-2127-2
Niks, M. and Otto, M., J. Immunol. Methods, 1990, vol. 130, no. 1, p. 149.
Matta, C.F. and Boyd, R.J., The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design, Wiley-VCH, 2007.
Espinosa, E., Molins, E., and Lecomte, C., Chem. Phys. Lett., 1998, vol. 285, p. 170.
Ananyev, I.V., Karnoukhova, V.A., Dmitrienko, A.O., and Lyssenko, K.A., J. Phys. Chem., 2017, vol. 121, p. 4517.
Puntus, L.N., Lyssenko, K.A., Antipin, M.Yu., et al., Inorg. Chem., 2008, vol. 47, p. 11095.
Borissova, A.O., Korlyukov, A.A., Antipin, M.Y., et al., J. Phys. Chem., 2008, vol. 112, p. 11519.
Vatsadze, S.Z., Medved’ko, A.V., Zyk, N.V., et al., Organometallics, 2009, vol. 28, p. 1027.
Abdulaeva, I.A., Birin, K.P., Sinelshchikova, A.A., et al., CrystEngComm, 2019, vol. 21, p. 1488.
Lyssenko, K.A., Mendeleev Commun., 2012, vol. 22, no. 1, p. 1.
Xanthopoulou, M.N., Hadjikakou, S.K., Hadjiliadis, N., et al., Inorg. Chem., 2007, vol. 46, p. 1187.
Ghosh, J. and Myers, C.E., Biochem. Biophys. Res. Commun., 1997, vol. 235, no. 2, p. 418.
Ghosh, J. and Myers, C.E., PNAS, 1998, vol. 95, no. 22, p. 13182.
Werz, O. and Steinhilber, D., Pharmacol. Ther., 2006, vol. 112, no. 3, p. 701.
ACKNOWLEDGMENTS
XRD studies were carried out on the equipment of the Center for Collective Use at the Department of Chemistry of the Moscow State University purchased in the framework of the Moscow University development program.
Funding
This work was supported by the Russian Science Foundation, project no. 22-23-00295.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Berseneva, D.A., Shpakovsky, D.B., Nikitin, E.A. et al. Anticancer Activity of New Organotin Complexes with Heterocyclic Thioamides. Russ J Coord Chem 49, 622–630 (2023). https://doi.org/10.1134/S1070328423600559
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600559