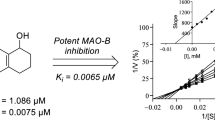
Abstract
The first ever synthesis of complexes [PdLCl2] (I) and [PdLBr2] (II) was successfully achieved, where L = 2,6-dimethyl-4-nitro-N-(pyridin-2-ylmethylildene)aniline, a ligand with a purported ability to inhibit monoamine oxidase B (MAO-B). To gain insight into the molecular structure of complexes I and II, as well as the ligand precursor 2,6-dimethyl-4-nitroaniline L4 (CIF files CCDC nos. 2255106 (I), 2255105 (II), 2255103 (L), 2255104 (L4)), X-ray diffraction analysis was utilized. Complex I underwent further characterization to determine its stability, solubility, and lipophilicity. Cytotoxicity studies of substances L, I, and II on human embryonic kidney cell line HEK-293 showed no evidence of cytotoxic activity. To evaluate the inhibitory activity of new substances L, I, and II as well as established substances III−IX, selegiline, and rasagiline, ex vivo studies were conducted, establishing a structure/activity relationship.




Similar content being viewed by others
REFERENCES
Ndagi, U., Mhlongo, N., and Soliman, M.E., Drug Des. Dev. Ther., 2017, vol. 11, p. 599. https://doi.org/10.2147/DDDT.S119488
Kotieva, I.M., Dodokhova, M.A., Safronenko, A.V., et al., J. Clin. Oncol., 2022, vol. 40, no. 16, p. e15080. https://doi.org/10.1200/JCO.2022.40.16_suppl.e15080
Yambulatov, D.S., Lutsenko, I.A., Nikolaevskii, S.A., et al., Molecules, 2022, vol. 27, no. 23, p. 8565. https://doi.org/10.3390/Molecules27238565
Czarnomysy, R., Radomska, D., Szewczyk, O.K., et al., Int. J. Mol. Sci., 2021, vol. 22, no. 15, p. 8271. https://doi.org/10.3390/ijms22158271
Abu-Surrah, A.S. and Kettunen, M., Curr. Med. Chem., 2006, vol. 13, no. 11, p. 1337.
Sharma, N.K., Ameta, R.K., and Singh, M., Biochem. Res. Int., 2016, vol. 2016. 4359375. https://doi.org/10.1155/2016/4359375
Scattolin, Th., Voshkin, V.A., Visentin, F., and Nolan, S.P., Cell. Rep. Phys. Sci., 2021, vol. 2, p. 100446. https://doi.org/10.1016/j.xcrp.2021.100446
Boyarskii, V.P., Mikherdov, A.S., Baikov, S.V., et al., Pharm. Chem. J., 2021, vol. 55, no. 2, p. 130. https://doi.org/10.1007/s11094-021-02393-1
Batyrenko, A.A., Mikolaichuk, O.V., Ovsepyan, G.K., et al., Russ. J. Gen. Chem., 2021, vol. 91, no. 4, p. 666. https://doi.org/10.1134/S1070363221040149
Zalevskaya, O.A., Gur’eva, Y.A., and Kutchin, A.V., Inorg. Chim. Acta, 2021, vol. 527, p. 120593. https://doi.org/10.1016/j.ica.2021.120593
Ibatullina, M.R., Zhil’tsova, E.P., Kulik, N.V., et al., Russ. Chem. Bull., 2022, vol. 71, no. 2, p. 314. https://doi.org/10.1007/s11172-022-3413-6
Denisov, M.S. and Glushkov, V.A., Bull. Perm. Univ. Chem., 2018, vol. 8, no. 4, p. 388. https://doi.org/10.17072/2223-1838-2018-4-388-411
Egorova, K.S., Galushko, A.S., and Ananikov, V.P., Angew. Chem., Int. Ed. Engl., 2020, vol. 59, p. 22296. https://doi.org/10.1002/anie.20200308
Denisov, M.S., Vestn. Perm. Feder. Issl. Tsentr., 2021, no. 4, p. 6. https://doi.org/10.7242/2658-705X/2021.4.1
Patra, M. and Gasse, G., ChemBioChem, 2012, vol. 13, no. 9, p. 1232. https://doi.org/10.1002/cbic.201200159
Özbek, N., Alyar, S., Memmi, B.K., et al., J. Mol. Struct., 2017, vol. 1127, p. 437. https://doi.org/10.1016/j.molstruc.2016.07.122
Ahmed, M., Khan, Sh.Z., Sher, N., et al., J. Venomous Anim. Toxins Incl. Trop. Dis., 2021, vol. 27, p. e20200047. https://doi.org/10.1590/1678-9199-JVATITD-2020-0047
Bal, S., Demirci, Ö., Şen, B., et al., Polyhedron, 2021, vol. 198, p. 115060. https://doi.org/10.1016/j.poly.2021.115060
Sahin, Ö., Özdemir, Ü.Ö., Seferoğlu, N., et al., J. Biomol. Struct. Dyn., 2021, p. 4460. https://doi.org/10.1080/07391102.2020.1858163
García-García, A., Rojas, S., Rivas-García, L., et al., Chem. Commun., 2022, vol. 58, p. 1514. https://doi.org/10.1039/D1CC04404D
Karataş, M.O., Çalgın, G., Alıcı, B., et al., Appl. Organomet. Chem., 2019, vol. 33, no. 10, p. e5130. https://doi.org/10.1002/aoc.5130
Asma, M., Badshah, A., Ali, S., et al., Transition Met. Chem., 2006, vol. 31, p. 556. https://doi.org/10.1007/s11243-006-0027-z
Lassig, J.P., Shultz, M.D., Gooch, M.G., et al., Arch. Biochem. Biophys., 1995, vol. 322, no. 1, p. 119. https://doi.org/10.1006/abbi.1995.1443
Vieites, M., Smircich, P., Parajón-Costa, B., et al., J. Biol. Inorg. Chem., 2008, vol. 13, no. 10, p. 1839. https://doi.org/10.1016/j.jinorgbio.2008.05.010
Fricker, S.P., Mosi, R.M., Cameron, B.R., et al., J. Inorg. Biochem., 2008, vol. 102, no. 10, p. 1839. https://doi.org/10.1016/j.jinorgbio.2008.05.010
Carneiro, Z.A., Lima, J.C., Lopes, C.D., et al., Eur. J. Med. Chem., 2019, vol. 180, no. 15, p. 213. https://doi.org/10.1016/j.ejmech.2019.07.014
Gama, N.H., Elkhadir, A.Y.F., Gordhan, B.G., et al., Biometals, 2016, vol. 29, p. 637. https://doi.org/10.1007/s10534-016-9940-6
Chen, Ch., Sun, L.-Yu., Gao, H., et al., ACS Infect. Dis., 2020, vol. 6, no. 5, p. 975. https://doi.org/10.1021/acsinfecdis.9b00385
Mital, R., Shah, G.M., Srivastava, T.S., and Bhattacharya, R.K., Life Sci., 1992, vol. 50, no. 11, p. 781. https://doi.org/10.1016/0024-3205(92)90183-P
Petrović, Z.D., Hadjipavlou-Litina, D., Pontiki, E., et al., Bioorg. Chem., 2009, vol. 37, no. 5, p. 162. https://doi.org/10.1016/j.bioorg.2009.07.003
Hegazy, W.H. and Al-Faiyz, Ya.S., Med. Chem. Res., 2014, vol. 23, no. 1, p. 518. https://doi.org/10.1007/s00044-013-0661-x
Lima, M.A., Costa, V.A., Franco, M.A., et al., Inorg. Chem. Commun., 2020, vol. 112, p. 107708. https://doi.org/10.1016/j.inoche.2019.107708
Krinulović, K., Bugarčić, Ž., Vrvić, M., et al., Toxicol. In Vitro, 2006, vol. 20, no. 8, p. 1292. https://doi.org/10.1016/j.tiv.2006.03.002
Tatyanenko, L.V., Kotelnikova, R.A., Zakharova, I.A., and Moshkovskii, Yu.Sh., Inorg. Chim. Acta, 1981, vol. 56, p. 89.
Parrilha, G.L., Ferraz, K.S.O., Lessa, J.A., et al., Eur. J. Med. Chem., 2014, vol. 84, no. 12, p. 537. https://doi.org/10.1016/j.ejmech.2014.07.055
Türkan, F., Huyut, Z., and Atalar, M.N., J. Biochem. Mol. Toxicol., 2018, vol. 32, no. 10, p. e22205, https://doi.org/10.1002/jbt.22205
Edmondson, D.E., Binda, C., and Mattevi, A., Arch. Biochem. Biophys., 2007, vol. 464, p. 269. https://doi.org/10.1016/j.abb.2007.05.006
Pharmaceutical Chemistry, Watson, D.R., Ed., Glasgow: Elsevier, 2011, p. 641.
Hong, R. and Li, X., MedChemComm, 2019, vol. 10, p. 10. https://doi.org/10.1039/c8md00446c
Tat’yanenko, L.V., Sokolova, N.V., and Mosh-kovsky, Y.S., Vopr. Med. Khim., 1982, vol. 28, p. 126.
Albert, J., Cadena, J.M., González, A., et al., Chem. Commun., 2003, vol. 41, no. 4, p. 528. https://doi.org/10.1039/B211808D
Cho, H.-U., Kim, S., Sim, J., et al., Exp. Mol. Med., 2021, vol. 53, p. 1148. https://doi.org/10.1038/s12276-021-00646-3
Denisov, M.S., Gagarskikh, O.N., and Utushkina, T.A., Bull. Perm. Univ. Chem., 2021, vol. 11, no. 1, p. 30. https://doi.org/10.17072/2223-1838-2021-1-30-58
Denisov, M.S., Dmitriev, M.V., Eroshenko, D.V., et al., Russ. J. Inorg. Chem., 2019, vol. 64, no. 1, p. 56. https://doi.org/10.1134/S0036023619010054
Yang, D.D., Wang, R., Zhu, J.L., et al., J. Mol. Struct., 2017, vol. 1128, no. 15, p. 493. https://doi.org/10.1016/j.molstruc.2016.08.037
Hao, Ch., Huang, W., Li, X., et al., Eur. J. Med. Chem., 2017, vol. 131, p. 1. https://doi.org/10.1016/j.ejmech.2017.02.063
CrysAlisPro. Agilent Technologies. Version 1.171.37.33 (release 27-03-2014 CrysAlis171 .NET).
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, p. 112. https://doi.org/10.1107/S0108767307043930
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Gonçalves, B.M.F., Salvador, J.A.R., Marín, S., and Cascante, M., Eur. J. Med. Chem., 2016, vol. 114, p. 101. https://doi.org/10.1016/j.ejmech.2016.02.057
Thull, U. and Testa, B., Biochem. Pharmacol., 1994, vol. 47, no. 22, p. 2307. https://doi.org/10.1016/0006-2952(94)90271-2
Andrade, J.M.M., Passos, C.D.S., Dresch, R.R., et al., Pharmacogn. Mag., 2014, vol. 10, no. 37, p. 100. https://doi.org/10.4103/0973-1296.127354
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., J. Biol. Chem., 1951, vol. 193, no. 1, p. 265.
O’Donnell, A.D., Gavriel, A.G., Christie, W., et al., Arkivoc, 2021, Pt. VI, p. 222. https://doi.org/10.24820/ark.5550190.p011.581
Park, S., Lee, J., Jeong, J.H., et al., Polyhedron, 2018, vol. 151, no. 1, p. 82. https://doi.org/10.1016/j.poly.2018.05.031
Motswainyana, W.M., Onani, M.O., Jacobs, J., and Meervelt, L.V., Acta Crystallogr. Sect. C: Cryst. Struct. Commun., 2012, vol. 68, p. 356. https://doi.org/10.1107/S0108270112045970
Laine, T.V., Klinga, M., and Leskelä, M., Eur. J. Inorg. Chem., 1999, vol. 1999, no. 6, p. 959. https://doi.org/10.1002/(SICI)1099-0682(199906)1999:6<959::AID-EJIC959>3.0.CO;2-Z
Delogu, G.L., Pintus, F., Mayán, L., et al., MedChemComm, 2017, vol. 8, p. 1788. https://doi.org/10.1039/C7MD00311K
Finberg, J.P.M. and Rabey, J.M., Front. Pharmacol., 2016, vol. 18, no. 7, p. 340. https://doi.org/10.3389/fphar.2016.00340
Denisov, M.S. and Gagarskikh, O.N., Russ. J. Gen. Chem., 2021, vol. 91, no. 7, p. 1354. https://doi.org/10.1134/S1070363221070136
ACKNOWLEDGMENTS
The work was carried out using the equipment of The Core Facilities Centre “Research of materials and matter” at the PFRC UB RAS. The author acknowledges O.A. Maiorova (Perm Federal Research Center, Ural Branch, RAS) for recording the NMR spectra, I.A. Borisova (Perm Federal Research Center, Ural Branch, RAS) for recording the IR spectra, M.V. Dmitriev for X-ray (Perm State University), A.A. Gorbunov for GC-MS and A.O. Voronina and O.N. Gagarskikh (Perm Federal Research Center, Ural Branch, RAS) for performing the MTT test.
Funding
The study was supported by the Russian Foundation for Basic Research and the Perm Region Ministry of Education and Science according to the Research Project no. 19-43-590003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest
Rights and permissions
About this article
Cite this article
Denisov, M.S., Beloglazova, Y.A. Nitro-Substituted Pyridinimine Complexes of Pd(II): Synthesis and Inhibition of MAO-B ex vivo. Russ J Coord Chem 49, 565–576 (2023). https://doi.org/10.1134/S1070328423600626
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600626