Abstract
Biofertilization is a technique that uses plant and animal wastes to add organic matter and nutrients to the soil. It can also use microorganisms that can metabolize these by-products to facilitate their absorption by the plant roots. In this study, we tested the effects of rhizospheric bacteria inoculation (T1), a combination of rhizospheric bacteria with liquid fertilizer (T2) and uncombined liquid fertilizer (T3), on the growth, nutritional content, root tissue, and root cells of lettuce plants. The results showed significant positive differences in all treatments compared to control plants, in terms of morphological, nutritional, and productivity parameters. The combination of rhizospheric bacteria with liquid SEFEL fertilizer (T2) yielded the best results, showing increased fresh and dry weight, and diameter. There were no differences between treatments for nutritional content, but each treatment outperformed the control by more than 700% for all macronutrients. The best result was phosphorus content for T1, with 1272.22% more than control. Regarding root structure and ultrastructure, there was no variation in tissue organization compared to control plants, but increases in root hairs (T1), development of transfer cells (T2), and secondary growth (T3) were observed. Additionally, colonization of roots by rhizospheric bacteria was confirmed in all three treatments. In conclusion, this study suggests that inoculating with rhizospheric bacteria is a viable and environmentally friendly biofertilization for lettuce plants.
Similar content being viewed by others
1 Introduction
Soil plays an essential role in terrestrial ecosystems, serving as a primary source of agricultural production. The functioning of soil is crucial for maintaining global biochemical cycles of essential nutrients. Agricultural practices have a significant impact on soil structure, function, and ecosystem balance (Kopittke et al. 2019). Fertilization is one such practice that involves adding nutrients to the soil in a form that can be assimilated by plants. However, the long-term consequences of fertilization can be detrimental to both social and environmental systems. These consequences can include soil salinization, degradation, desertification, loss of fertility, and contamination of aquifers, among others (Matzeu et al. 2017; Buvaneshwari et al. 2020; Rastgoo and Hasanfard 2021). Therefore, exploration of alternative and sustainable agricultural practices that can mitigate the negative impact of fertilization on soil and the environment is under way (Sharma & Chetani 2017).
One such alternative is biofertilization, which involves the use of plant remains and livestock manure to provide the soil with organic matter and nutrients. It can include the use of microorganisms capable of metabolizing these by-products, thus facilitating their absorption by the plant root system (Wei et al. 2020). In addition, some microorganisms used as or in biofertilizers have other important functions, such as acting as plant growth promoters. This is through the synthesis of phytohormones that produce changes in plant tissue structure (Noh-Medina et al. 2014; Guimarães et al. 2022), the mobilization of nutrients (Pandey and Yarzábal 2019), induction of systemic resistance (Meena et al. 2020), improvement of soil particle structure and aggregation through the production of siderophores, (Rani et al. 2023; Nerling et al. 2022), or by the inhibition capacity of other phytopathogenic microorganisms (Pandey and Yarzábal 2019). Among these latter, Bacillus and Pseudomonas genera stand out (Khan et al. 2021; Guzmán-Guzmán and Santoyo 2022). For all such reasons, biofertilizers are much more effective when several microorganisms are used in combination (Anli et al. 2020).
Commercial biofertilizers based on microorganisms generally consist of one or two of these. The most used are those belonging to the two genera last mentioned, Bacillus and Pseudomonas, as well as the genera Azospirillum, Rhizobium and Rhizobacter or the mycorrhizae of various fungal species. In addition, within the wide variety of biofertilizers available, there are those of the SEFEL type (from the Spanish acronym: Sistema de Elaboración de Fertilizantes Líquidos Ecológicos) (Acosta 2013). These are easy to apply to crops; they regenerate soils and facilitate nutrient absorption by plant roots (Hernández 2015).
By using biofertilizers, it is possible to provide structure to the soil, prevent its degradation, and increase mineralization processes by microorganisms. These benefits lead to an increase in soil fertility and crop productivity in relatively short periods, while decreasing the use of fossil fuel energy and pollution of ecosystems (Asoegwu et al. 2020). In addition, these microorganisms can cause beneficial structural and tissue changes in plants, such as an increase in adventitious roots, general root growth, stem elongation, increased vascular cylinder development, formation of transfer cells, etc. (Garay-Arroyo et al. 2014; Hakim et al. 2021).
The main objective of this work was to test the effect of three experimental treatments on the development, yield, and nutritional content of Iceberg lettuce plants (Lactuca sativa L. var. capitata). These treatments were: inoculation of rhizospheric bacteria, application of a SEFEL-type liquid fertilizer and the combined application of the first two. The bacteria used were isolated from a healthy, developed crop of this plant. In addition, we studied how these treatments affected the root tissues and cells and what changes are induced, at light and electron microscopy level, with special attention to the presence of microorganisms in root ultrastructure after soil inoculation.
2 Materials and methods
2.1 Soil used in the study
Lettuce cultivation was initiated in pots with soil taken from the Higher Polytechnic School of Engineering, University of La Laguna, Canary Islands, Spain (28º28’42.65” N, 16º19’08.68” W; altitude 564 m above sea level). To determine its physicochemical characteristics prior to the trials, a soil sample was dried at room temperature, then sieved (2 mm gauge) and the following parameters were analyzed: pH, electrical conductivity in the saturation extract, organic matter, Olsen’s phosphorus, assimilable Ca, Mg, Na and K (MAGRAMA 1994).
2.2 Isolation of rhizospheric microorganisms
Samples of rhizospheric material, including root remains, were collected from five points around healthy lettuce plants on a farm. The selected lettuces met the criteria of being of appropriate size and appearance for their species, having abundant green leaves and stems, good root development, and being free of malformations, pests, and diseases. At each of the sampling points, approximately 50 g of soil and roots were taken at a depth of 10–30 cm. Each of these points conformed a subsample, pooled into a sample of about 250 g (Nath et al. 2019). These were transferred to the laboratory in sealed sterile plastic bags and refrigerated.
From the pooled samples, the roots together with the soil aggregates were crushed under sterile conditions using a pestle and mortar, to homogenize the sample and allow the extraction of endophytic microorganisms, if present. From these treated samples, serial dilutions were made with saline solution (0.85% NaCl), inoculating Petri dishes with 100 µL of each in the optimal media used to isolate the types of microorganism sought. Microorganisms were selected according to their nitrogen fixation and phosphorus solubilization, as well as their capacity to synthesize phytohormones or their precursors. Pseudomonas sp. were isolated on Cetrimide agar (Scharlab, Spain), endospore-forming bacteria on Tryptone-Soya agar (TSA) (Scharlab, Spain) after subjecting dilutions to heat shock at 80 °C for 12 min (Nihorimbere and Ongena 2017). In turn, phosphorus-solubilizing microorganisms were isolated on Pikovskayas agar (Saravanan et al. 2015), and free-living diazotrophs on Ashby medium (Cañón et al. 2009).
Five colonies of the morphotypes obtained in each culture medium were subjected to an antagonism test by the confrontation technique on nutrient agar (Undabarrena et al. 2016). This determined whether any of the selected microorganisms inhibited the growth of others, discarding them from further use. In addition, we subjected the strains to a hemolysis assay on blood agar to exclude those that might be pathogenic to humans (Mendonça et al. 2022). The selected microorganisms were cryopreserved at -20 ºC in 20% glycerol before use.
2.3 Preparation of bacterial inocula
The selected microorganisms were grown in a sterile extract of the recipient soil, enriched with glucose and yeast extract. To prepare this, 300 g of soil and roots were homogenized in the same way as for species isolation. This homogenate was added to 1 L of distilled water and shaken for one hour, allowed to stand for 30 min at room temperature and centrifuged at 3,8000 g for 15 min. The supernatant obtained was filtered through filter paper and autoclaved at 121 °C for 15 min. Then, 300 mL of soil extract, 1.6 g of yeast extract, and 0.66 g of glucose were added to 1 L of distilled water at room temperature (Hamaki et al. 2005). With this medium, we attempted to imitate natural growth conditions in the surrounding rhizosphere, to optimize microbial adaptation to the soil.
This medium was inoculated and incubated at 26 °C under continuous agitation for 24–48 h, until it presented a McFarland turbidity of 0.5 (108 CFU mL− 1). It was then kept in the refrigerator until use, for a period never exceeding 24 h.
2.4 Liquid fertilizer (SEFEL)
The System for the Elaboration of Liquid Ecological Fertilizers (SEFEL) produces compost tea-type fertilizers from agricultural and livestock by-products subjected to aerobic fermentation, resulting in an organic liquid product (Acosta 2013). Such a biofertilizer (hereafter SEFEL) contains few minerals and microorganisms, but regenerates soils by augmenting their chemical, physical and microbiological fertility, which is reflected in crop production. Its use for irrigation allows saline soils in particular to regenerate and reach nutrient balance over time. SEFEL not only increases productivity, but also saves an average of 30% in water consumption. It is also of great interest in island regions like the Canaries, as it reduces dependence on external inputs and thus reduces costs. (Hernández 2015). The fertilizer used in the assays presented the physicochemical characteristics and microbiological content shown in Tables 1 and 2.
2.5 Lettuce sowing and inoculation
The assay was carried out in pots prepared with soil and a layer of gravel at the bottom for drainage. Lettuce seedlings (Lactuca sativa L. var capitata) with the same degree of development were taken from the seedbed and planted in these pots. Each trial had control pots and pots in which we applied the treatments below (T1, T2 and T3),
-
Control: 150 mL of irrigation water.
-
T1: 50 mL of inoculated soil extract and 100 mL of irrigation water.
-
T2: 30 mL of inoculated soil extract, 20 mL of SEFEL and 100 mL of irrigation water.
-
T3: 20 mL of SEFEL + 130 mL of irrigation water.
In treatments T1 and T2, the inoculated soil extract was only added once at the beginning of the trial, while in treatments T2 and T3 SEFEL was added weekly. Pots were randomly distributed in a greenhouse where the trials were conducted. Light intensity conditions were measured with a Quantum Meter and were generally 700–800 µmol m− 2 s− 1. Temperature was about 23–25ºC.
Pots were drip irrigated to maintain stable soil humidity conditions. To facilitate seedling rooting, the pot was initially saturated to field capacity. For T2 and T3, besides daily drip irrigation, we supplied additional water to dilute SEFEL during its application once a week, to avoid plant damage, Therefore, even though the control and T1 pots did not need this addition, as drip irrigation was sufficient, they received more water to equalize conditions. In all treatments the final volume of liquid was 150 mL.
2.6 Morphological, chemical, and nutritional analysis of lettuce plants
To check the effect of the different treatments on the growth and nutritional content of the treated lettuces, the following parameters were analyzed:
-
Diameter and length were determined using a tape measure before harvesting. Two measurements were taken and averaged, one longitudinal and one transverse.
-
Fresh and dry weight after two weeks growth. Plants with each treatment from each block were cut at ground level without including the root, placed individually in a bag and taken to the laboratory. They were then weighed on a digital weighing machine to obtain the fresh weight. After this, each lettuce stem was cut longitudinally to ensure better drying, and they were kept in an oven at 80ºC for 6 days. After this time, they were cooled in a desiccator, weighed to determine the dry weight.
-
Nutritional characteristics of lettuce plants were determined following the laboratory techniques described in Métodos Oficiales de Análisis (MAGRAMA 1994). The dried lettuce samples were ground in an IKA M20 micromill and placed in properly identified envelopes. They were kept in an oven at 105 °C for five hours, then transferred to a desiccator and finally weighed. Nitrogen was determined by the Kjeldahl method. A 0.1 g sample of dry material was taken for this purpose. For the nutrients Ca, Mg, Na, K, P, Cu, Fe, Mn, and Zn, 1 g of ground powder was taken from each leaf sample, which had been mineralized dry with 6 N hydrochloric acid, after acenization in a muffle furnace at 480 °C. The elements were then determined in a Perkin Elmer ICP, model Optima 2000D from this 1 g extract.
2.7 Morphological and ultrastructural studies of roots
Samples of lettuce roots (Lactuca sativa L. var. capitata) were obtained when the plants were harvested two weeks after trial commencement. One pot was randomly selected from each of the three treatments (T1, T2, and T3) and one from the control, thus obtaining four samples.
The roots hairs of the lettuce were washed in water carefully. Once as clean as possible, 2–3 mm cross sections (c.s.) were made, separating the root apices from the middle sections. These apical and middle c.s. were processed by light microscopy (LM) and transmission electron microscopy (TEM), using standard protocols. For each treatment (T1, T2, and T3), ten apical and 20 medium portions were obtained, and the same in the control.
Small root pieces (2–3 mm) were fixed in 3% glutaraldehyde in 0.1 M phosphate buffer (PB) for 2 h, degassing after the first hour, post-fixing in 1% OsO4, in PB 0.1 M for 2 h. They were then dehydrated in an ethanol gradient series, transferred to propylene oxide, and embedded in Spurr’s resin (Spurr 1969). Semi-thin (0.5-1 μm) and ultra-thin Sect. (70 nm) were cut using a Reicher-Jung ultramicrotome. Semi-thin sections were stained with toluidine blue, examined, and photographed using a Leica Mod. D4000B light microscope. Ultra-thin sections were stained with uranyl acetate and lead citrate. These sections were photographed in a JEM-1010 TEM (Singular Scientific and Technological Infraestructures (ICTS), Microscopy, Complutense University of Madrid) and in a JEOL JEM-1010 transmission electron microscope (General Services for Supporting Research, University of La Laguna).
2.8 Statistical analysis
The data obtained were analyzed using the “Data analysis” add-in of the Microsoft Office Excel 2007 program. A 2-way analysis of variance (2-Way ANOVA) was performed, and differences were considered significant at P < 0.05.
3 Results and discussion
3.1 Soil chemical analysis and bacterial inocula
The total bacterial content in the inocula used after 48 h of incubation at 26 °C was 2.31 × 1010 CFU mL− 1. The soil where the lettuces were grown presented suitable chemical characteristics for this crop, showing slightly alkaline pH, medium organic matter content and low salinity indexes. The absolute exchangeable potassium content was high, but assimilable phosphorus was somewhat low (Table 3). Phosphorus is a structural and functional component of nucleic acids, phospholipids, energy metabolites and activated intermediates in the photosynthetic carbon cycle. Furthermore, inorganic phosphate plays a crucial role in signal transduction cascades (Wang et al. 2021). Transformation of mineral or non-exchangeable P to plant-assimilable forms can occur by geochemical, but mainly biological processes (Hou et al. 2018). Therefore, the use of phosphorus-potassium solubilizing bacteria can mobilize this nutrient without the need for external inputs (Meena et al. 2016).
3.2 Morphological and productive parameters of lettuce plants
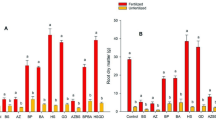
The fresh and dry weights of the lettuce plants in the treatments were always higher than in the control plants (p < 0.05), the highest values being in the inoculated extract treatment combined with SEFEL (T2). For this treatment, the fresh weight of the lettuces was 42.73% higher than that of the control plants, and the dry weight was 47.03% higher. These results were followed in fresh and dry weight by those treated with the inoculated extract (T1) and with SEFEL (T3), showing no significant differences between these treatments. The lowest weights were reached by the control (Table 4).
The first measurements of plant diameters showed no significant differences between treatments. However, after 15 days, the largest diameters were those of the treatments with the inoculated extract (T1) and its combination with SEFEL (T2), with an increase over control of 17.96% and 19.42% respectively. Thus, the values for these treatments were within the same statistical category and showed clear significant differences (P < 0.05) from T3 and the controls (Table 4).
The significant differences between the treated plants with respect to the control plants reflect the benefits of applying the two fertilization methods. The plants that underwent T2 (inoculated extract + SEFEL) reached higher fresh and dry weights than the others (p < 0.05). However, in terms of plant size there were no significant differences from T1. It should be noted that the significant differences between treatments (a, b, and c) were the same for fresh and dry weight, ruling out the possibility that the benefit of these treatments lies only in an increase in the water content of the tissues.
These increases in size and weight of the plants subjected to treatments T1 and T2 were probably determined by the bacterial action and contribution of organic matter, since the content of nutrients such as N increased (Table 5). Indeed, N is an element closely related to biomass production (Peng et al. 2019). It could also be due to the direct and indirect mechanisms of plant growth promotion by the inoculated microorganisms, such as phytohormone production (Stefen et al. 2022) or nutrient solubilization (Asghar et al. 2023). In the case of T3 (SEFEL), the results may have been inferior because organic fertilizers generally release minerals slowly, which is favored by microbial action. Despite being a fertilizer based on microorganisms, its joint action with the inoculated extract (T2) can accelerate the mineralization and humification processes and therefore soil nutrients availability (Li et al. 2022).
In other trials using microorganisms as biofertilizers in various plant species, similar results have been obtained, increasing fresh and dry weights, diameter, leaf area, etc. with respect to controls (Noh-Medina et al. 2014; Kalayu 2019; Kumar and Sandhya 2022). The relationship between improvements in these parameters and increased nutrient content is illustrated and explained in the following section.
3.3 Nutritional content of lettuce plants
Macronutrients Ca, Mg, K, and P were higher in all treatments, and all three were significantly different from the control, but not from each other. (Table 5). They always increased over control by more than 700%. The best result was at T1 for P content, with 1272% above control. Considering the low levels of assimilable P in the soil used for the crop (Table 4), this increase could be due to the solubilization of mineral or non-assimilable P (Meena et al. 2016). The microorganisms responsible for this phenomenon can colonize and solubilize rocks by various mechanisms. These include the production of organic acids that rapidly dissolve rock, releasing assimilable P into the soil. They can also produce Si ion chelators, which indirectly have the same effect (Alyousif 2022). In addition, rhizospheric bacteria can form polysaccharide capsules that increase adherence to mineral particles. They accumulate organic acids, accelerating mineral solubilization (Liu et al. 2012). Bacterial and fungal species can also associate to form biofilms, in which several solubilization mechanisms combine to accelerate mineral degradation and nutrient mobilization towards plants (Lucero et al. 2023).
The elements studied also showed differences in favor of the treatments with inoculated extract (T1), with SEFEL (T2) and the combination (T3), when compared to control (Table 5). These nutrient levels in control lettuces were below the standards cited (Hartz et al. 2007), particularly P. This could be because they only received irrigation water without nutrient solution, as recommended by Ezziddine et al. (2021) for hydroponic crops. However, the lettuces submitted to the treatments were found to be within the normal nutritional ranges.
Nitrogen content was higher in the treatment with inoculated extract (T1), followed by that with SEFEL (T3), but did not differ significantly from the combined treatment (T2), which in turn not differ significantly different from the control treatment, unlike T1 and T3 (Table 3).
The macronutrients listed in Table 5 are not only necessary for plant development, but also have several beneficial effects on plants. Nitrogen favors vegetative growth, which is of interest in crops such as lettuce, providing succulence to the plant (Benett et al. 2019). On the other hand, Ca, like P, contributes to root development, which indirectly leads to a greater development of the aerial part of the plant, stimulating P especially in the seedling stage. (Álzate et al. 2019).
Finally, K and Mg are important elements in photosynthesis, enzyme activation, and synthesis of organic molecules, Mg being a fundamental component of chlorophyll molecules (Zepka et al. 2019). In general, higher concentrations of these macronutrients in plants produce beneficial effects, while their deficiency can lead to disease and poor plant development. However, they must be perfectly balanced, as they may have synergistic effects on some and antagonistic effects on others. An example is P, which inhibits the absorption of Ca, Zn and K, reducing the assimilation of the latter also because of high N concentrations. In addition, K can inhibit the assimilation of B and Mg, but favors that of Mn and Fe (Thomson and Troeh 1982; Symanowicz 2020; Xie et al. 2021).
The micronutrients listed in Table 5, despite being found in plants in much lower concentrations than macronutrients, are also necessary for their correct development. These elements do not usually show synergy with each other, although they often have antagonistic effects. Thus, Mn contributes to metabolic processes of great importance in plant cells, such as photosynthesis and N assimilation through the roots; however it can inhibit Fe absorption (Rai et al. 2021). Iron can in turn inhibit the absorption of N, in addition to P and Cu, and is important for its nitrate and sulfate reducing capacity, as well as being part of several pigments and enzymes. Both Zn and Cu are responsible for the activation of enzymes involved in the synthesis of certain proteins, carbohydrates, lignin and even chlorophyll. However, Zn can inhibit absorption of other transition metals like Cu, Mn, and Fe (Rai et al. 2021). Finally, B was lower in all treatments compared to control, although not significantly. This may be due to the high K content, which inhibits B uptake (Symanowicz 2020). Boron is essential for the formation of the cell wall in synergy with Ca and therefore for cell division and growth, in addition to favoring absorption of Mg, as an exception to the rarity of synergistic micronutrients (Mohebbi et al. 2020).
All treatments led to an improvement in nutrient assimilation and therefore nutrient content, especially limiting nutrient levels such as of N and P. These data highlight the benefits of applying rhizospheric microorganisms (Goicoechea and Antolín 2017) and SEFEL fertilizer, which also contains microorganisms (Table 2). The higher amounts of micro- and macronutrients in the plants receiving the two active treatments compared to control plants, with the resultant benefit, could be due to the significant increase in weight and diameter of the lettuces. However, on analyzing their nutritional contents, especially macronutrients, it is clear that T1 obtained the best results. Although no notable differences were observed when compared to T2 and T3, it is worth mentioning that T1 was only administered once at the beginning of the crop, in contrast to the SEFEL booster applied in the other treatments. This not only saves costs, but also reduces the time needed to administer the treatment.
3.4 Structure and ultrastructure of the roots of lettuce plants
Cross sections (c.s.) of control roots presented a concentric tissue structure, simple epidermis with some root hairs, parenchymatous cortex with isodiametric cells and scarce intercellular space, and vascular bundle arrangement in amphicribal haplostele (Fig. 1a). The vascular cylinder was delimited by the pericycle and a poorly defined endodermis (Fig. 1b). Longitudinal section of roots showed an apical meristem with euchromatic nucleus, nucleolus, and abundant vacuoles, protected by a cap of large cells containing starch deposits. The ultrastructure showed no evidence of colonization by microorganisms, or of disease.
Light microscope photomicrographs of a control plant root in cross section. 1a. Panoramic view, concentric arrangement of tissues, epidermis (E), cortical parenchyma (C) and amphicribal vascular bundle (VB). 1b. Cortical parenchyma delimited by epidermis (E) and endodermis (En); xylem (Xy) and phloem (Ph) surrounded by pericycle (Pe). a-b. graph scale 25 μm
The roots in c.s. of the three treatments, T1, T2, T3, showed a tissue distribution like those of the control, although with some variations. Ultrastructurally, root cell colonization by microorganisms was detected.
The root epidermis of T1 showed more abundant root hairs than in the control plants (data not quantified, but observable) (Fig. 2a and b), and the presence of prokaryotic microorganisms close to the wall and inside the epidermal cells (Figs. 2b and 3a g). These additional hairs expand the absorption surface area, enhancing water and nutrient assimilation (Dolan 2017). This may be due to the production of auxin-type phytohormones by the inoculated microorganisms, as demonstrated by Kurniawan & Chuang (2022) in Arabidopsis thaliana inoculated with Bacillus mycoides. These hormones regulate many aspects of plant development, especially the root, which is highly sensitive to them. Application of auxins augments root development in general, in itself increasing surface area for nutrient absorption and therefore greater development of the aerial part of the plant (Márquez et al. 2016; Vandepol et al. 2022). The parenchymatous cells (T1) were well developed with intact walls, living protoplast and endomembrane remnants (Fig. 2c and d). The conducting elements of the xylem showed a secondary wall with prokaryotic microorganisms visible in the lumen of some parenchyma cells associated with the vascular cylinder (Fig. 2d). The high level of colonization by these inoculated microorganisms could cause the improved nutritional content, plant growth and development (Bhatti and Thakur 2022). They are able to regulate nutrient availability in the rhizosphere (Castro-Barquero et al. 2015), since root development and consequently plant growth are directly determined by this immediate environment. Nutrient analysis (Table 5) was richer in plants submitted to this treatment (T1), as reflected in the increased fresh and dry weights of their aerial zone.
Light microscope photomicrographs of the root of T1 plants, in cross section. 2a. Panoramic view, concentric arrangement of tissues, epidermis (E), cortical parenchyma (C) and vascular bundle (VB). Abundant root hairs (RH). 2b. Epidermis and root hairs detail. a-b. graph scale 25 μm. 2c. Cortical cells with intact walls and protoplasmic content. 2d. Conductive elements of the xylem and parenchyma associated with the presence of microorganisms (arrow)
Transmission electron microscopy photomicrographs of T1 plant roots in cross section. 3a-3 g. Microorganisms inside and outside of the root cells. 3a. Epidermal cells, root hairs and microorganisms on the cell exterior. 3b-3c. Detail of microorganisms framed in 3a. 3d-f. Epidermal cells and microorganisms inside the cell. 3e. Detail of microorganisms framed in 3d. 3 g. Detail of microorganisms framed in 3 f
In the case of T2, in root c.s., granular content was visible in the cortical parenchyma cells, corresponding to prokaryotic microorganisms colonizing the root (Fig. 4a and b g). Electron-dense material accumulated in the lumen and these microorganisms presented a peribacterial-type membrane (Fig. 5a and b). Prokaryotic microorganisms were also found between the walls of two contiguous cells (Fig. 5c). Parenchymatous cell walls showed primary growth and in the living protoplast the nucleus and endomembrane remnants were observed (Fig. 4d). In addition, transfer cells were detected (Fig. 4e and f), characterized by a secondary thickening of the cell wall, giving rise to a labyrinth-like wall that significantly increases its surface area. This increase in surface area allows an increase in the number of transporters in the cell wall (Zhang et al. 2019). Transfer cells showed good communication between parenchyma cells, improving transport of nutrients along the whole root (Xia et al. 2020). These structures may appear because of colonization by symbiont or pathogenic microorganisms (Offler et al. 2003). Plant analysis confirmed the good nutritional status of the samples receiving this treatment, which points to a contribution by transfer cells.
Light and transmission electron microscope photomicrographs of T2 plant roots in cross section. 4a. Panoramic view, concentric arrangement of tissues, cortical parenchyma (C), vascular bundle (VB), xylem (Xy) and phloem (Ph) 4b. Detail of the region framed in 4a, parenchymal cells with granular content corresponding to prokaryotic microorganisms (*). 4c. Phloem and xylem elements in amphicribal arrangement. a-c. graph scale 25 μm. 4d. Primary cell wall (Fw), middle lamella (Lm) and nucleus (Nu) of a parenchymal cell. 4e-4 f. Transfer cell (Tc) with wall (Tcw) showing secondary labyrinth-like thickening. 4 g. Cortex cell with microorganisms (*)
Transmission electron microscope photomicrographs of a T2 plant root, in cross section. 5a. Cortical parenchymal cells with living protoplast and prokaryotic microorganisms. 5b. Detail of microorganisms surrounded by a membrane, such as the peribacterial-membrane. 5c. Detail of microorganisms passing between the walls of two adjoining cells
In roots of T3 plants, the vascular cylinder was notably more developed (Fig. 6a and b) with a vascular cambium, which is responsible for lateral growth (Nieminen et al. 2015). This produces secondary xylem inwards and secondary phloem outwards (Ragni and Greb 2018), resulting in a haplostele vascular pattern. It has been observed in Arabidopsis roots that some hormones, such as cytokinins, are required for cambial growth, since their absence causes non-activation of the cambium (Nieminem et al. 2015). Indeed, T3 with SEFEL could act as a growth promoter like the cytokinins, promoting development of the lateral meristem. The ultrastructural level revealed the presence of prokaryotic microorganisms (Fig. 7), which were surrounded by a peribacterial-type membrane or immersed in an amorphous structure with medium electron density (Fig. 7f). Plant-microorganism interaction was observed in the three treatments, and the physiological state of the bacteria seemed adequate after their addition to the soil. We could not confirm the entry mechanism of microorganisms in any of the treatments. The prokaryotic microorganisms detected in epidermal cells in T1 may have entered through root hairs or an alternative route, e.g., intercellularly between undamaged epidermal cells, as proposed by Troiani et al. (2017) and Sharma et al. (2020) in groundnut. Other authors propose that microcolonies could develop on the surface of epidermal cells and at cell junctions, where the mucilaginous layer that covers the root epidermis has less surface tension. This difference allows these endophytic bacteria to penetrate the intercellular regions, where there is more space and opportunity for their movement (Ragavi et al. 2019). Furthermore, as verified by Menéndez et al. (2020) with Mesorizhobium strains, some bacteria can produce cellulases and cellulose. This would facilitate degradation and restructuring of the cell wall to be colonized by these strains, allowing entry not only to the wall and intercellular junctions, but also to the cytoplasm. Prokaryotic microorganisms were also seen between walls of two contiguous cells (Fig. 5c) in T2, supporting intercellular propagation as occurs in the “crack-entry” strategy of Bradyrhizobium (Sharma et al. 2020). More studies are necessary. These bacteria contribute to increased efficiency of nutrient acquisition by the root and a greater development of the lettuce plant compared to controls.
Light microscope photomicrographs of the of a T3 plant root in cross section. 6a. Panoramic view of the vascular cylinder with secondary growth. Parenchymatous cortex (C), endodermis (En) and vascular bundle (VB). 6b. Detail of the elements of the xylem (Xy), the vascular cambium (VC) and the phloem (Ph). a-b. graph scale 25 μm
Transmission electron microscopy photomicrographs of a T3 plant root in cross section. 7a. Conductive and associated elements of the xylem (Xy). 7b. Detail of the region framed in 7a. Microorganisms in the cell lumen (*). 7c. Detail of the region framed in 7a. Walls with secondary growth (star) and microorganisms close to it. 7d. Detail of the region framed in 7a. Cell from xylem elements with euchromatic nucleus (Nu), vacuole (V) and wall with plasmodesmata (Pl). 7e. Xylem elements and vascular cambium zone. 7 f. Detail of the region framed in 7e; microorganisms (asterisk *). 7 g. Detail of the region framed in 7e. Xylem parenchyma cell with organelles (O) and microorganisms (*). 7 h. Detail of the region framed in 7e. Parenchymal cell of the cylinder with some organelles and microorganisms (*)
4 Conclusion
To conclude, the results of this study suggest that inoculation of rhizospheric bacteria isolated from mature lettuce may be a promising and environmentally friendly biofertilization option for this crop. Treatments T1 and T2 indeed showed better results in terms of size, fresh and dry weight, and nutrient content. Although we observed no variations in tissue organization in transverse sections of roots after each treatment, an increase in root hairs (T1), development of transfer cells (T2), and secondary growth with elongation of vascular bundles and vascular cambium (T3) were in fact detected. In addition, TEM confirmed root colonization by rhizospheric bacteria in all three treatments. Although hemolysis by the strains was ruled out, further studies are needed to ensure the safety of these microorganisms for human consumption. These findings provide valuable information for the development of more sustainable and environmentally friendly agricultural practices, which are crucial to ensure long-term food security and environmental protection.
References
Acosta I (2013) SEFEL (Sistema de Elaboración de Fertilizantes Ecológicos Líquidos). Patente Nº de solicitud de 201101258 y Nº de publicación ES2405532
Alyousif NA (2022) Review of genetic analysis and mechanisms of phosphate solubilization by phosphate solubilizing bacteria. Marsh Bull 17:8–22
Álzate J, Fonseca L, Muriel J (2019) Efecto de diferentes mezclas de nitrógeno, fósforo y potasio en el desarrollo y rendimiento del híbrido de maíz (Zea mays L) 19 – 10 (Fenalce) Yacuanquer, Nariño–Colombia. Rev Cienc Agropec 5(1):3–12. https://doi.org/10.36436/24223484.190
Anli M, Baslam M, Tahiri A, Raklami A, Symanczik S, Boutasknit A et al (2020) Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci 11:516818. https://doi.org/10.3389/fpls.2020.516818
Asghar I, Ahmed M, Gul I, Arshad M, Ishtiaq M, Mahmood RT et al (2023) Plant growth promotion and nutrient mobilization by indigenous bacteria from soil under long term wheat and maize cultivation in district Bhimber. Pak J Agric Sci, 60(1)
Asoegwu CR, Awuchi CG, Nelson KCT, Orji CG, Nwosu OU, Egbufor UC, Awuchi CG (2020) A review on the role of biofertilizers in reducing soil pollution and increasing soil nutrients. Hmlyn J Agr 1:34–38
Benett KSS, Xavier RC, Benett CGS, Salomão LC, Seleguini A, Cantuario FS, Martins AS (2019) Nitrogen application in arugula culture. J Agric Sci 11(2):385–391. https://doi.org/10.5539/jas.v11n2p385
Bhatti SK, Thakur M (2022) An overview on orchids and their interaction with endophytes. Bot Rev 88(4):485–504. https://doi.org/10.1007/s12229-022-09275-5
Buvaneshwari S, Riotte J, Sekhar M, Sharma AK, Helliwell R, Kumar MM et al (2020) Potash fertilizer promotes incipient salinization in groundwater irrigated semi-arid agriculture. Sci Rep 10(1):3691. https://doi.org/10.1038/s41598-020-60365-z
Cañón RF, Prato VP, Sabino MAA, Caro DMC (2009) Efecto del uso del suelo sobre rizobacterias fosfatosolubizadoras y diazotroficas en el distrito de riego del río Zulia, Norte de Santander (Colombia). Rptas 14(2):14–21
Castro-Barquero L, Murillo-Roos M, Lorío LU, Mata-Chinchilla R (2015) Inoculación al suelo con Pseudomonas fluorescens, Azospirillum oryzae, Bacillus subtilis y Microorganismos de Montaña (MM) y su efecto sobre un sistema de rotación soya-tomate bajo condiciones de invernadero. Agron Costarricense 39:21–36
Dolan L (2017) Root hair development in grasses and cereals (Poaceae). Curr Opin Genet Dev 45:76–81. https://doi.org/10.1016/j.gde.2017.03.009
Ezziddine M, Liltved H, Seljåsen R (2021) Hydroponic lettuce cultivation using organic nutrient solution from aerobic digested aquacultural sludge. Agron 11(8):1484
Garay-Arroyo A, de la Paz Sánchez M, García-Ponce B, Álvarez-Buylla ER, Gutiérrez C (2014) La homeostasis de las auxinas y su importancia en el desarrollo de Arabidopsis thaliana. Rev Educ Bioq 33(1):13–22
Goicoechea N, Antolín MC (2017) Increased nutritional value in food crops. Microb Biotechnol 10(5):1004–1007. https://doi.org/10.1111/1751-7915.12764
Guimarães GS, Rondina ABL, Santos MS, Nogueira MA, Hungria M (2022) Pointing out Opportunities to increase Grassland Pastures Productivity via Microbial Inoculants: attending the Society’s demands for meat production with sustainability. Agronomy 12(8):1748. https://doi.org/10.3390/agronomy12081748
Guzmán-Guzmán P, Santoyo G (2022) Action mechanisms, biodiversity, and omics approaches in biocontrol and plant growth-promoting Pseudomonas: an updated review. Biocontrol Sci Technol 32(5):527–550. https://doi.org/10.1080/09583157.2022.2066630
Hakim S, Naqqash T, Nawaz MS, Laraib I, Siddique MJ, Zia R et al (2021) Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front Sustain Food Syst 5:617157. https://doi.org/10.3389/fsufs.2021.617157
Hamaki T, Suzuki M, Fudou R, Jojima Y, Kajiura T, Tabuchi A et al (2005) Isolation of novel bacteria and actinomycetes using soil-extract agar medium. J Biosci Bioeng 99(5):485–492. https://doi.org/10.1263/jbb.99.485
Hartz TK, Johnstone PR, Williams E, Smith RF (2007) Establishing lettuce leaf nutrient optimum ranges through DRIS analysis. HortSci 42(1):143–146. https://doi.org/10.21273/HORTSCI.42.1.143
Hernández M (2015) Regeneración de un suelo con la aplicación de un té de compost obtenido mediante el Sistema de Elaboración de Fertilizantes Ecológicos Líquidos (SEFEL). XXIV Jornadas Técnicas de SEAE, I Jornadas Antonio Bello “Agroecología: Suelo vivo para una vida sana”, Tenerife
Hou E, Chen C, Luo Y, Zhou G, Kuang Y, Zhang Y et al (2018) Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob Chang Biol 24(8):3344–3356. https://doi.org/10.1111/gcb.14093
Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int. J. Agron. 2019:1–7. https://doi.org/10.1155/2019/4917256
Khan M, Salman M, Jan SA, Shinwari ZK (2021) Biological control of fungal phytopathogens: a comprehensive review based on Bacillus species. MOJ Biol Med 6:90–92. https://doi.org/10.15406/mojbm.2021.06.00137
Kopittke PM, Menzies NW, Wang P, McKenna BA, Lombi E (2019) Soil and the intensification of agriculture for global food security. Environ Int 132:105078. https://doi.org/10.1016/j.envint.2019.105078
Kumar AM, Sandhya GM (2022) Synergistic potential of microorganisms (bio-fertilizer) on growth performance of Meliadubia. Ecol Environ Conserv 28:352–359. https://doi.org/10.53550/EEC.2022.v28i04s.054
Kurniawan A, Chuang HW (2022) Rhizobacterial Bacillus mycoides functions in stimulating the antioxidant defence system and multiple phytohormone signalling pathways to regulate plant growth and stress tolerance. J Appl Microbiol 132(2):1260–1274.https://doi.org/10.1111/jam.15252
Li H, Zhang T, Shaheen SM, Abdelrahman H, Ali EF, Bolan NS et al (2022) Microbial inoculants and struvite improved organic matter humification and stabilized phosphorus during swine manure composting: Multivariate and multiscale investigations. Bioresour Technol 351:126976. https://doi.org/10.1016/j.biortech.2022.126976
Liu D, Lian B, Dong H (2012) Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol J 29(5):413–421. https://doi.org/10.1080/01490451.2011.576602
Lucero CT, Lorda GS, Halliday N, Ambrosino ML, Cámara M, Taurian T (2023) Impact of quorum sensing from native peanut phosphate solubilizing Serratia sp. S119 strain on interactions with agronomically important crops. Symbiosis 89(1):107–121. https://doi.org/10.1007/s13199-022-00893-6
MAGRAMA (1994) Métodos Oficiales de Análisis, tomo III. Ministerio de Agricultura, Pesca y Alimentación. Secretaría General de alimentación. Dirección general de Política Alimentaria, Madrid
Márquez G, Alarcón MV, Salguero J (2016) Differential responses of primary and lateral roots to indole-3-acetic acid, indole-3-butyric acid, and 1-naphthaleneacetic acid in maize seedlings. Biol Plant 60(2):367–375. https://doi.org/10.1007/s10535-015-0576-0
Matzeu A, Secci R, Uras G (2017) Methodological approach to assessment of groundwater contamination risk in an agricultural area. Agric Water Manag 184:46–58. https://doi.org/10.1016/j.agwat.2017.01.003
Meena V, Bahadur I, Maurya B, Kumar A, Meena R, Meena S, Verma J (2016) Potassium-solubilizing microorganism in evergreen agriculture: an overview. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 1–20. https://doi.org/10.1007/978-81-322-2776-2_1
Meena M, Swapnil P, Divyanshu K, Kumar S, Tripathi YN, Zehra A et al (2020) PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: current perspectives. J Basic Microbiol 60(10):828–861. https://doi.org/10.1002/jobm.202000370
Mendonça FS, Navarro MA, Uzal FA (2022) The comparative pathology of enterocolitis caused by Clostridium perfringens type C, Clostridioides difficile, Paeniclostridium sordellii, Salmonella enterica subspecies enterica serovar Typhimurium, and nonsteroidal anti-inflammatory drugs in horses. J Vet. Diagn. Invest. 34(3):412–420. https://doi.org/10.1177/10406387211041091
Menéndez E, Pérez-Yépez J, Hernández M, Rodríguez-Pérez A, Velázquez E, León-Barrios M (2020) Plant growth promotion abilities of phylogenetically diverse Mesorhizobium strains: effect in the root colonization and development of tomato seedlings. Microorganisms 8(3):412. https://doi.org/10.3390/microorganisms8030412
Mohebbi S, Babalar M, Zamani Z, Askari MA (2020) Influence of early season boron spraying and postharvest calcium dip treatment on cell-wall degrading enzymes and fruit firmness in ‘Starking Delicious’ apple during storage. Sci Hortic 259:108822. https://doi.org/10.1016/j.scienta.2019.108822
Nath S, Paul P, Roy R, Bhattacharjee S, Deb B (2019) Isolation and identification of metal-tolerant and antibiotic-resistant bacteria from soil samples of Cachar district of Assam, India. SN Appl Sci 1:1–9. https://doi.org/10.1007/s42452-019-0762-3
Nerling D, Castoldi CT, Ehrhardt-Brocardo NCM (2022) The role of PGPR-Polar Metabolites, Metal-Chelator Compounds and Antibiotics on Plant Growth. In: Sayyed RZ, Uarrota VG (eds) Secondary metabolites and volatiles of PGPR in Plant-Growth Promotion. Springer, Cham, pp 119–131. https://doi.org/10.1007/978-3-031-07559-9_5
Nieminen K, Blomster T, Helariutta Y, Mähönen AP (2015) Vascular cambium development. Arabidopsis Book 13:1–23. https://doi.org/10.1199/tab.0177
Nihorimbere V, Ongena M (2017) Isolation of Plant growth-promoting Bacillus strains with Biocontrol Activity in vitro. Merit Res J Microbiol Biol Sci 5(2):13–21
Noh-Medina J, Yam-Chimal C, Borges-Gómez L, Zúñiga-Aguilar J, Godoy-Hernández G (2014) Aislados bacterianos con potencial biofertilizante para plántulas de tomate. Terra Latinoam 32(4):273–281
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2003) Transfer cells: cells specialized for a special purpose. Annu Rev Plant Biol 54(1):431–454. https://doi.org/10.1146/annurev.arplant.54.031902.134812
Pandey A, Yarzábal LA (2019) Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments. Appl Microbiol Biotechnol 103(2):643–657. https://doi.org/10.1007/s00253-018-9515-2
Peng Y, Peng Z, Zeng X, Houx JH (2019) Effects of nitrogen-phosphorus imbalance on plant biomass production: a global perspective. Plant Soil 436:245–252. https://doi.org/10.1007/s11104-018-03927-5
Ragavi G, Muthamilan M, Nakkeeran S, Kumaravadivel N, Sivakumar U, Suganthi A (2019) Phenotypic and molecular characterization of endophytic Bacteria isolated from Banana. Curr J Appl Sci Technol 38(6):1–10. https://doi.org/10.9734/cjast/2019/v38i630402
Ragni L, Greb T (2018) Secondary growth as a determinant of plant shape and form. Semin Cell Dev Biol 79:58–67. https://doi.org/10.1016/j.semcdb.2017.08.050
Rai S, Singh PK, Mankotia S, Swain J, Satbhai SB (2021) Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 1:100008. https://doi.org/10.1016/j.stress.2021.100008
Rani AA, Basha SM, Darsha KD, Christy CA, Nagaiah HP, Kasthuri T, Pandian SK (2023) Plant growth promoting Rhizobacteria and their biofilms in promoting sustainable agriculture and soil health. In: Das S, Kungwani NA (eds.) Understanding Microbial Biofilms. Academic Press, pp 629–647. https://doi.org/10.1016/B978-0-323-99977-9.00003-X
Rastgoo M, Hasanfard A (2021) Desertification in agricultural lands: approaches to mitigation. In: Zhu Y, Luo Q, Liu Y (eds) Deserts and desertification. IntechOpen, London, pp 153–166. https://doi.org/10.5772/intechopen.82931
Saravanan D, Radhakrisha M, Balagurunathan R (2015) Bioprospecting of bacteria from less explored ecosystem. J Chem Pharm Res 7(3):852–857
Sharma A, Chetani R (2017) A review on the effect of organic and chemical fertilizers on plants. Int J Res Appl Sci Eng Technol 5:677–680
Sharma V, Bhattacharyya S, Kumar R, Kumar A, Ibañez F, Wang J et al (2020) Molecular basis of the root nodules symbiosis between Bradyrhizobium and ‘crack-entry’ legume groundnut (Arachis hypogaea L). Plants 9:276. https://doi.org/10.3390/plants9020276
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26(1–2):31–43. https://doi.org/10.1016/S0022-5320(69)90033-1
Stefen DLV, Nunes FR, Rodolfo GR, Segatto C, Anastácio TC, Lajus CR (2022) How phytohormones synthesized by PGPR affect Plant Growth? In: Sayyed RZ, Uarrota VG (eds) Secondary metabolites and volatiles of PGPR in Plant-Growth Promotion. Springer, Cham, pp 119–131. https://doi.org/10.1007/978-3-031-07559-9
Symanowicz B (2020) Antagonistic changes in the content of molybdenum and boron in field pea and in soil under of the influence potassium fertilisation. J Elem 25(1). https://doi.org/10.5601/jelem.2019.24.4.1897
Thomson L, Troeh L (1982) Los suelos y su fertilidad. Editorial Reverté, Barcelona
Troiani HO, Prina AO, Muiño WA, Tamame MA, Beinticinco L (2017) Botánica, morfología, taxonomía y fitogeografía. EdUNLPam, Santa Rosa
Undabarrena A, Beltrametti F, Claverías FP, González M, Moore ER, Seeger M, Cámara B (2016) Exploring the diversity and antimicrobial potential of marine actinobacteria from the comau fjord in Northern Patagonia. Chile Front Microbiol 7:1135. https://doi.org/10.3389/fmicb.2016.01135
Vandepol N, Liber J, Yocca A, Matlock J, Edger P, Bonito G (2022) Linnemannia elongata (Mortierellaceae) stimulates Arabidopsis thaliana aerial growth and responses to auxin, ethylene, and reactive oxygen species. PLoS ONE 17(4):e0261908. https://doi.org/10.1371/journal.pone.0261908
Wang Y, Chen YF, Wu WH (2021) Potassium and phosphorus transport and signaling in plants. J Integr Plant Biol 63(1):34–52. https://doi.org/10.1111/jipb.13053
Wei M, Zhang M, Huang G, Yuan Y, Fu C, Yu L (2020) Coculture with two Bacillus velezensis strains enhances the growth of Anoectochilus plants via promoting nutrient assimilation and regulating rhizosphere microbial community. Ind Crops Prod 154:112697. https://doi.org/10.1016/j.indcrop.2020.112697
Xia X, Zhang HM, Offler CE, Patrick JW (2020) Enzymes contributing to the hydrogen peroxide signal dynamics that regulate wall labyrinth formation in transfer cells. J Exp Bot 71(1):219–233. https://doi.org/10.1093/jxb/erz443
Xie K, Cakmak I, Wang S, Zhang F, Guo S (2021) Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J 9(2):249–256. https://doi.org/10.1016/j.cj.2020.10.005
Zepka LQ, Jacob-Lopes E, Roca M (2019) Catabolism and bioactive properties of chlorophylls. Curr Opin Food Sci 26:94–100. https://doi.org/10.1016/j.cofs.2019.04.004
Zhang HM, Devine LB, Xia X, Offler CE, Patrick JW (2019) Ethylene and hydrogen peroxide regulate formation of a sterol-enriched domain essential for wall labyrinth assembly in transfer cells. J Exp Bot 70(5):1469–1482
Acknowledgements
We gratefully acknowledge the technical assistance of the Electron Microscopy Service (ICTS) of the Complutense University of Madrid and the SEGAI of the University of La Laguna, Tenerife. Also, thanks to Guido Jones for language revision.
Funding
This work was supported by the aid program of the Consejería de Economía, Industria, Comercio y Conocimiento for training research staff in the Canary Islands, co-financed by the Fondo Social Europeo (grant numbers: TESIS2020010011, TESIS2017010078). We also declare funding from Nertalab S.L.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology and supervision by CAC, MHG and NAA; data collection by CAC, ZEA, MHG; data analysis by DTM and CAC; writing, review and editing by DMF, CAC, EHB and ZEA; manuscript preparation by DMF and CAC; project design by CAC, MHG and NAA; administration, and funding acquisition by CAC, MHG and NAA. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare they have no conflicts of interest.
Additional information
Communicated by Yurina Kwack
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montesdeoca-Flores, D., Alfayate-Casañas, C., Hernández-Bolaños, E. et al. Effect of biofertilizers and rhizospheric bacteria on growth and root ultrastucture of lettuce. Hortic. Environ. Biotechnol. 65, 15–28 (2024). https://doi.org/10.1007/s13580-023-00545-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-023-00545-8