Abstract
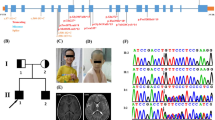
Intellectual disability (ID), occurring in syndromic or non-syndromic forms, is the most common neurodevelopmental disorder. Although many cases are caused by single gene defects, ID is highly genetically heterogeneous. Biallelic variants in the transmembrane protein TMEM147 have recently been linked to intellectual disability with dysmorphic facial features. TMEM147 is believed to localize to the endoplasmic reticulum membrane and nuclear envelope and also involved in biogenesis of multi-pass membrane proteins. Here, we report two patients born to a consanguineous family with a novel loss-of-function variant; (NM_001242597.2:c.193-197del) in TMEM147 causing intellectual disability and spasticity. Whole exome sequencing and validating Sanger sequencing were utilized to confirm the identified causal variant. Our findings were in line with the previously described patients with TMEM147 variants manifesting intellectual disability as a major clinical sign but also featured spasticity as a phenotypic expansion. This study provides additional evidence for the pathogenicity of TMEM147 mutations in intellectual disability and expands the phenotypic and variant spectrum linked to this gene.


Similar content being viewed by others
Change history
20 September 2023
A non-academic email was mistakenly used by Dr. Hossein Mazdarani (co-corresponding author) in the proof. This has been replaced with his academic email.
References
Harripaul R, Noor A, Ayub M, Vincent JB (2017) The use of next-generation sequencing for research and diagnostics for intellectual disability. Cold Spring Harb Perspect Med 7(3). https://doi.org/10.1101/cshperspect.a026864
Hu H, Kahrizi K, Musante L, Fattahi Z, Herwig R, Hosseini M, Oppitz C, Abedini SS, Suckow V, Larti F, Beheshtian M (2019) Genetics of intellectual disability in consanguineous families. Mol Psychiatry 24(7):1027–1039. https://doi.org/10.1038/s41380-017-0012-2
Reuter MS et al (2017) Diagnostic yield and novel candidate genes by exome sequencing in 152 consanguineous families with neurodevelopmental disorders. JAMA Psychiatry 74(3):293–299. https://doi.org/10.1001/jamapsychiatry.2016.3798
Dettmer U, Kuhn PH, Abou-Ajram C et al (2010) Transmembrane protein 147 (TMEM147) is a novel component of the Nicalin-NOMO protein complex. J Biol Chem 285(34):26174–26181. https://doi.org/10.1074/jbc.M110.132548
Rosemond E, Rossi M, McMillin SM, Scarselli M, Donaldson JG, Wess J (2011) Regulation of M3 muscarinic receptor expression and function by transmembrane protein 147. Mol Pharmacol 79(2):251–261. https://doi.org/10.1124/mol.110.067363
Christodoulou A, Maimaris G, Makrigiorgi A et al (2020) TMEM147 interacts with lamin B receptor, regulates its localization and levels, and affects cholesterol homeostasis. J Cell Sci 133(16). https://doi.org/10.1242/jcs.245357
Duband-Goulet I, Courvalin JC, Buendia B (1998) LBR a chromatin and lamin binding protein from the inner nuclear membrane, is proteolyzed at late stages of apoptosis. J Cell Sci 111(10):1441–1451. https://doi.org/10.1242/jcs.111.10.1441
Koczok K, Gurumurthy CB, Balogh I, Korade Z, Mirnics K (2019) Subcellular localization of sterol biosynthesis enzymes. J Mol Histol 50(1):63–73. https://doi.org/10.1007/s10735-018-9807-y
McGilvray PT, Anghel SA, Sundaram A et al (2020) An ER translocon for multi-pass membrane protein biogenesis. Elife 9. https://doi.org/10.7554/eLife.56889
Thomas Q, Motta M, Gautier T et al (2022) Bi-allelic loss-of-function variants in TMEM147 cause moderate to profound intellectual disability with facial dysmorphism and pseudo-Pelger-Huët anomaly. Am J Hum Genet 109(10):1909–1922. https://doi.org/10.1016/j.ajhg.2022.08.008
Sundaram A, Yamsek M, Zhong F, Hooda Y, Hegde RS, Keenan RJ (2022) Substrate-driven assembly of a translocon for multipass membrane proteins. Nature 611(7934):167–172. https://doi.org/10.1038/s41586-022-05330-8
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26(5):589–595. https://doi.org/10.1093/bioinformatics/btp698
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164. https://doi.org/10.1093/nar/gkq603
Karczewski KJ, Francioli LC, Tiao G et al (2021) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 590(7846):E53. https://doi.org/10.1038/s41586-020-2308-7
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Oosterwijk JC, Mansour S, van Noort G, Waterham HR, Hall CM, Hennekam RC (2003) Congenital abnormalities reported in Pelger-Huët homozygosity as compared to Greenberg/HEM dysplasia: highly variable expression of allelic phenotypes. J Med Genet 40(12):937–941. https://doi.org/10.1136/jmg.40.12.937
Hoffmann K, Dreger CK, Olins AL et al (2002) Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huët anomaly). Nat Genet 31(4):410–414. https://doi.org/10.1038/ng925
Bolar NA, Golzio C, Živná M et al (2016) Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet 99(1):174–187. https://doi.org/10.1016/j.ajhg.2016.05.028
Graham HK et al (2015) Cerebral palsy. Nat Rev Dis Prim 2(1):15082. https://doi.org/10.1038/nrdp.2015.82
Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N (2018) The complex aetiology of cerebral palsy. Nat Rev Neurol 14(9):528–543. https://doi.org/10.1038/s41582-018-0043-6
Michael-Asalu A, Taylor G, Campbell H et al (2019) Cerebral palsy: diagnosis, epidemiology, genetics, and clinical update. Adv Pediatr 66:189–208. https://doi.org/10.1016/j.yapd.2019.04.002
Radio FC, Pang K, Ciolfi A et al (2021) SPEN haploinsufficiency causes a neurodevelopmental disorder overlapping proximal 1p36 deletion syndrome with an episignature of X chromosomes in females. Am J Hum Genet 108(3):502–516. https://doi.org/10.1016/j.ajhg.2021.01.015
Acknowledgements
We thank all family members for their participation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Student Research Committee (SRC) of the Faculty of Medical Sciences, Tarbiat Modares University.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghorashi, T., Darvish, H., Bakhtiari, S. et al. A biallelic loss-of-function variant in TMEM147 causes profound intellectual disability and spasticity. Neurogenetics 24, 311–316 (2023). https://doi.org/10.1007/s10048-023-00734-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-023-00734-8