Abstract
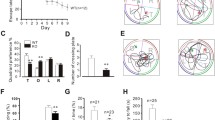
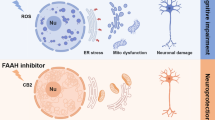
Heart failure (HF) is a major health burden worldwide, with approximately half of HF patients having a comorbid cognitive impairment (CI). However, it is still unclear how CI develops in patients with HF. In the present study, a mice model of heart failure was established by ligating the left anterior descending coronary artery. Echocardiography 1 month later confirmed the decline in ejection fraction and ventricular remodeling. Cognitive function was examined by the Pavlovian fear conditioning and the Morris water maze. HF group cued fear memory, spatial memory, and learning impairment, accompanied by activation of glial cells (astrocytes, microglia, and oligodendrocytes) in the hippocampus. In addition, the mitochondrial biogenesis genes TFAM and SIRT1 decreased, and the fission gene DRP1 increased in the hippocampus. Damaged mitochondria release excessive ROS, and the ability to produce ATP decreases. Damaged swollen mitochondria with altered morphology and aberrant inner-membrane crista were observed under a transmission electron microscope. Finally, Akt/mTOR signaling was upregulated in the hippocampus of heart failure mice. These findings suggest that activation of Akt/mTOR signaling, glial activation, and mitochondrial dynamics imbalance could trigger cognitive impairment in the pathological process of heart failure mice.





Similar content being viewed by others
Data Availability
Under appropriate request to the corresponding author.
References
Adams J (1988) Ashburn Hall. N Z Med J 101(850):491
Akomolafe A, Quarshie A, Jackson P, Thomas J, Deffer O, Oduwole A, Onwuanyi A, Lapu-Bula R, Strayhorn G, Ofili E, Mayberry R (2005) The prevalence of cognitive impairment among African-American patients with congestive heart failure. J Natl Med Assoc 97(5):689–694
Alers S, Löffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32(1):2–11. https://doi.org/10.1128/mcb.06159-11
Althammer F, Ferreira-Neto HC, Rubaharan M, Roy RK, Patel AA, Murphy A, Cox DN, Stern JE (2020) Three-dimensional morphometric analysis reveals time-dependent structural changes in microglia and astrocytes in the central amygdala and hypothalamic paraventricular nucleus of heart failure rats. J Neuroinflammation 17(1):221. https://doi.org/10.1186/s12974-020-01892-4
Baburina Y, Krestinin R, Fedorov D, Odinokova I, Pershina E, Sotnikova L, Krestinina O (2022) The improvement of functional state of brain mitochondria with astaxanthin in rats after heart failure. Int J Mol Sci 24(1). https://doi.org/10.3390/ijms24010031
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58(4):495–505. https://doi.org/10.1002/ana.20624
Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH (2007) mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem 87(2):303–307. https://doi.org/10.1016/j.nlm.2006.08.007
Bélanger M, Magistretti PJ (2009) The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11(3):281–295. https://doi.org/10.31887/DCNS.2009.11.3/mbelanger
Belelovsky K, Kaphzan H, Elkobi A, Rosenblum K (2009) Biphasic activation of the mTOR pathway in the gustatory cortex is correlated with and necessary for taste learning. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience 29(23):7424–7431. https://doi.org/10.1523/jneurosci.3809-08.2009
Bennett SJ, Cordes DK, Westmoreland G, Castro R, Donnelly E (2000) Self-care strategies for symptom management in patients with chronic heart failure. Nurs Res 49(3):139–145. https://doi.org/10.1097/00006199-200005000-00004
Cadonic C, Sabbir MG, Albensi BC (2016) Mechanisms of mitochondrial dysfunction in Alzheimer’s disease. Mol Neurobiol 53(9):6078–6090. https://doi.org/10.1007/s12035-015-9515-5
Campbell CT, Kolesar JE (1819) Kaufman BA (2012) Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochem Biophys Acta 9–10:921–929. https://doi.org/10.1016/j.bbagrm.2012.03.002
Chen Z, Trapp BD (2016) Microglia and neuroprotection. J Neurochem 136(Suppl 1):10–17. https://doi.org/10.1111/jnc.13062
Chu F, Li K, Li X, Xu L, Huang J, Yang Z (2021) Graphene oxide ameliorates the cognitive impairment through inhibiting PI3K/Akt/mTOR pathway to induce autophagy in AD mouse model. Neurochem Res 46(2):309–325. https://doi.org/10.1007/s11064-020-03167-z
Deli A, Schipany K, Rosner M, Höger H, Pollak A, Li L, Hengstschläger M, Lubec G (2012) Blocking mTORC1 activity by rapamycin leads to impairment of spatial memory retrieval but not acquisition in C57BL/6J mice. Behav Brain Res 229(2):320–324. https://doi.org/10.1016/j.bbr.2012.01.017
Dobson GP, Morris JL, Letson HL (2022) Immune dysfunction following severe trauma: a systems failure from the central nervous system to mitochondria. Front Med 9:968453. https://doi.org/10.3389/fmed.2022.968453
El-Hattab AW, Scaglia F (2016) Mitochondrial Cardiomyopathies Frontiers in Cardiovascular Medicine 3:25. https://doi.org/10.3389/fcvm.2016.00025
Evonuk KS, Prabhu SD, Young ME, DeSilva TM (2017) Myocardial ischemia/reperfusion impairs neurogenesis and hippocampal-dependent learning and memory. Brain Behav Immun 61:266–273. https://doi.org/10.1016/j.bbi.2016.09.001
Finsterer J (2012) Cognitive dysfunction in mitochondrial disorders. Acta Neurol Scand 126(1):1–11. https://doi.org/10.1111/j.1600-0404.2012.01649.x
Frey A, Popp S, Post A, Langer S, Lehmann M, Hofmann U, Sirén AL, Hommers L, Schmitt A, Strekalova T, Ertl G, Lesch KP, Frantz S (2014) Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain. Front Behav Neurosci 8:376. https://doi.org/10.3389/fnbeh.2014.00376
Frey A, Homola GA, Henneges C, Mühlbauer L, Sell R, Kraft P, Franke M, Morbach C, Vogt M, Müllges W, Ertl G, Solymosi L, Pirpamer L, Schmidt R, Pham M, Störk S, Stoll G (2021) Temporal changes in total and hippocampal brain volume and cognitive function in patients with chronic heart failure-the COGNITION.MATTERS-HF cohort study. Eur Heart J 42(16):1569–1578. https://doi.org/10.1093/eurheartj/ehab003
Frobert A, Valentin J, Cook S, Lopes-Vicente J, Giraud MN (2014) Cell-based therapy for heart failure in rat: double thoracotomy for myocardial infarction and epicardial implantation of cells and biomatrix. J Vis Exp (91):51390. https://doi.org/10.3791/51390
Gaita F, Corsinovi L, Anselmino M, Raimondo C, Pianelli M, Toso E, Bergamasco L, Boffano C, Valentini MC, Cesarani F, Scaglione M (2013) Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol 62(21):1990–1997. https://doi.org/10.1016/j.jacc.2013.05.074
Ge Y, Xu W, Zhang L, Liu M (2020) Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1β in central nervous system. Int Immunopharmacol 85:106652. https://doi.org/10.1016/j.intimp.2020.106652
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49. https://doi.org/10.1016/j.pneurobio.2013.10.004
Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A (2017) Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther 23(1):5–22. https://doi.org/10.1111/cns.12655
Harkness K, Heckman GA, Akhtar-Danesh N, Demers C, Gunn E, McKelvie RS (2014) Cognitive function and self-care management in older patients with heart failure. Eur J Cardiovasc Nurs 13(3):277–284. https://doi.org/10.1177/1474515113492603
Hong X, Bu L, Wang Y, Xu J, Wu J, Huang Y, Liu J, Suo H, Yang L, Shi Y, Lou Y, Sun Z, Zhu G, Behnisch T, Yu M, Jia J, Hai W, Meng H, Liang S, Huang F, Zou Y, Ge J (2013) Increases in the risk of cognitive impairment and alterations of cerebral β-amyloid metabolism in mouse model of heart failure. PloS One 8(5):e63829. https://doi.org/10.1371/journal.pone.0063829
Iadecola C (2013) The pathobiology of vascular dementia. Neuron 80(4):844–866. https://doi.org/10.1016/j.neuron.2013.10.008
Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S (1996) A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224(3):855–862. https://doi.org/10.1006/bbrc.1996.1112
Jara C, Aranguiz A, Cerpa W, Tapia-Rojas C, Quintanilla RA (2018) Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol 18:279–294. https://doi.org/10.1016/j.redox.2018.07.010
Joshi AU, Minhas PS, Liddelow SA, Haileselassie B, Andreasson KI, Dorn GW 2nd, Mochly-Rosen D (2019) Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci 22(10):1635–1648. https://doi.org/10.1038/s41593-019-0486-0
Karbowski M, Neutzner A (2012) Neurodegeneration as a consequence of failed mitochondrial maintenance. Acta Neuropathol 123(2):157–171. https://doi.org/10.1007/s00401-011-0921-0
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3):151–166. https://doi.org/10.1016/j.tins.2017.01.002
Kim DI, Lee KH, Gabr AA, Choi GE, Kim JS, Ko SH, Han HJ (2016) Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochem Biophys Acta 1863 11:2820–2834. https://doi.org/10.1016/j.bbamcr.2016.09.003
Kittleson MM (2022) A clinician’s guide to the 2022 ACC/AHA/HFSA guideline for the management of heart failure. J Cardiac Fail 28(5):831–834. https://doi.org/10.1016/j.cardfail.2022.03.346
Koirala S, Guo Q, Kalia R, Bui HT, Eckert DM, Frost A, Shaw JM (2013) Interchangeable adaptors regulate mitochondrial dynamin assembly for membrane scission. Proc Natl Acad Sci USA 110(15):E1342-1351. https://doi.org/10.1073/pnas.1300855110
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration 9(1):42. https://doi.org/10.1186/s40035-020-00221-2
Lesuis SL, Catsburg LAE, Lucassen PJ, Krugers HJ (2018) Effects of corticosterone on mild auditory fear conditioning and extinction; role of sex and training paradigm. Learning & Memory (cold Spring Harbor, NY) 25(10):544–549. https://doi.org/10.1101/lm.047811.118
Li H, Liu Y, Lin LT, Wang XR, Du SQ, Yan CQ, He T, Yang JW, Liu CZ (2016) Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats. Neurochem Int 92:35–42. https://doi.org/10.1016/j.neuint.2015.12.001
Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC, Cohen RM, Zhang Q (2017) Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging 49:165–182. https://doi.org/10.1016/j.neurobiolaging.2016.10.003
Lu Z, Yang T, Wang L, Qiu Q, Zhao Y, Wu A, Li T, Cheng W, Wang B, Li Y, Yang J, Zhao M (2020) Comparison of different protocols of Morris water maze in cognitive impairment with heart failure. Brain behav 10(2):e01519. https://doi.org/10.1002/brb3.1519
Lu Z, Teng Y, Wang L, Jiang Y, Li T, Chen S, Wang B, Li Y, Yang J, Wu X, Cheng W, Cui X, Zhao M (2022) Abnormalities of hippocampus and frontal lobes in heart failure patients and animal models with cognitive impairment or depression: a systematic review. PloS One 17(12):e0278398. https://doi.org/10.1371/journal.pone.0278398
Luo RY, Luo C, Zhong F, Shen WY, Li H, Hu ZL, Dai RP (2020) ProBDNF promotes sepsis-associated encephalopathy in mice by dampening the immune activity of meningeal CD4(+) T cells. J Neuroinflammation 17(1):169. https://doi.org/10.1186/s12974-020-01850-0
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK (2022) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Revista Espanola De Cardiologia (english Ed) 75(6):523. https://doi.org/10.1016/j.rec.2022.05.005
McMurray JJ, Pfeffer MA (2005) Heart failure. Lancet (london, England) 365(9474):1877–1889. https://doi.org/10.1016/s0140-6736(05)66621-4
Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, Topisirovic I (2015) mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle (georgetown, Tex) 14(4):473–480. https://doi.org/10.4161/15384101.2014.991572
Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, Pearl D, Siddiqui N, Strack S, McGuirk S, St-Pierre J, Larsson O, Topisirovic I, Vali H, McBride HM, Bergeron JJ, Sonenberg N (2017) mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol Cell 67(6):922-935.e925. https://doi.org/10.1016/j.molcel.2017.08.013
National Clinical Guideline C (2010) National institute for health and clinical excellence: guidance. Chronic heart failure: national clinical guideline for diagnosis and management in primary and secondary care: partial update. London: Royal College of Physicians (UK) Copyright © 2010, National Clinical Guideline Centre
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. https://doi.org/10.1146/annurev-immunol-032713-120240
Palzur E, Edelman D, Sakas R, Soustiel JF (2021) Etifoxine restores mitochondrial oxidative phosphorylation and improves cognitive recovery following traumatic brain injury. Int J Mol Sci 22(23). https://doi.org/10.3390/ijms222312881
Park JS, Davis RL, Sue CM (2018) Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep 18(5):21. https://doi.org/10.1007/s11910-018-0829-3
Rajeev V, Fann DY, Dinh QN, Kim HA, De Silva TM, Lai MKP, Chen CL, Drummond GR, Sobey CG, Arumugam TV (2022) Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics 12(4):1639–1658. https://doi.org/10.7150/thno.68304
Roger VL (2013) Epidemiology of heart failure. Circ Res 113(6):646–659. https://doi.org/10.1161/circresaha.113.300268
Russo E, Citraro R, Constanti A, De Sarro G (2012) The mTOR signaling pathway in the brain: focus on epilepsy and epileptogenesis. Mol Neurobiol 46(3):662–681. https://doi.org/10.1007/s12035-012-8314-5
Russo E, Leo A, Crupi R, Aiello R, Lippiello P, Spiga R, Chimirri S, Citraro R, Cuzzocrea S, Constanti A, De Sarro G (2016) Everolimus improves memory and learning while worsening depressive- and anxiety-like behavior in an animal model of depression. J Psychiatr Res 78:1–10. https://doi.org/10.1016/j.jpsychires.2016.03.008
Salehi P, Shahmirzadi ZY, Mirrezaei FS, Shirvani Boushehri F, Mayahi F, Songhori M, Abofazeli M, Motaghinejad M, Safari S (2019) A hypothetic role of minocycline as a neuroprotective agent against methylphenidate-induced neuronal mitochondrial dysfunction and tau protein hyper-phosphorylation: possible role of PI3/Akt/GSK3β signaling pathway. Med Hypotheses 128:6–10. https://doi.org/10.1016/j.mehy.2019.04.017
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23. https://doi.org/10.1007/s12035-012-8344-z
Schieke SM, Finkel T (2006) Mitochondrial signaling, TOR, and life span. Biol Chem 387(10–11):1357–1361. https://doi.org/10.1515/bc.2006.170
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682. https://doi.org/10.1038/nmeth.2019
Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, Polovina M, Gale CP, Lund LH, Lopatin Y, Lainscak M, Savarese G, Huculeci R, Kazakiewicz D, Coats AJS (2021) The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail 23(6):906–914. https://doi.org/10.1002/ejhf.2143
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87(4):387–406
Simmons EC, Scholpa NE, Schnellmann RG (2020) Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp Neurol 329:113309. https://doi.org/10.1016/j.expneurol.2020.113309
Stockburger C, Miano D, Pallas T, Friedland K, Muller WE (2016) Enhanced neuroplasticity by the metabolic enhancer piracetam associated with improved mitochondrial dynamics and altered permeability transition pore function. Neural Plast 2016:8075903–8075903
Su J, Wang J, Ma Y, Li Q, Yang Y, Huang L, Wang H, Li H, Wang Z, Tong J, Huang D, Bai X, Yu M, Bu L, Fei J, Huang F (2019) Inflammation associated with chronic heart failure leads to enhanced susceptibility to depression. FEBS J 286(14):2769–2786. https://doi.org/10.1111/febs.14839
Su C, Xiao Y, Zhang G, Liang L, Li H, Cheng C, Jin T, Bradley J, Peberdy MA, Ornato JP, Mangino MJ, Tang W (2022) Exogenous nicotinamide adenine dinucleotide attenuates postresuscitation myocardial and neurologic dysfunction in a rat model of cardiac arrest. Crit Care Med 50(2):e189–e198. https://doi.org/10.1097/ccm.0000000000005268
Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM (2002) A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA 99(1):467–472. https://doi.org/10.1073/pnas.012605299
Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS (2018) Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Failure 6(8):678–685. https://doi.org/10.1016/j.jchf.2018.03.006
Wang HW, Ahmad M, Jadayel R, Najjar F, Lagace D, Leenen FHH (2019) Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction. PloS One 14(6):e0217437. https://doi.org/10.1371/journal.pone.0217437
Wang S, Chen Y, Li X, Zhang W, Liu Z, Wu M, Pan Q, Liu H (2020) Emerging role of transcription factor EB in mitochondrial quality control. Biomed Pharmacother 128:110272. https://doi.org/10.1016/j.biopha.2020.110272
Xu F, Na L, Li Y, Chen L (2020) Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10(1):54. https://doi.org/10.1186/s13578-020-00416-0
Yahya T, Jilani MH, Khan SU, Mszar R, Hassan SZ, Blaha MJ, Blankstein R, Virani SS, Johansen MC, Vahidy F, Cainzos-Achirica M, Nasir K (2020) Stroke in young adults: current trends, opportunities for prevention and pathways forward. Am J Prev Cardiol 3:100085. https://doi.org/10.1016/j.ajpc.2020.100085
Yang T, Lu Z, Wang L, Zhao Y, Nie B, Xu Q, Han X, Li T, Zhao J, Cheng W, Wang B, Wu A, Zhu H, Meng W, Shang H, Zhao M (2020) Dynamic changes in brain glucose metabolism and neuronal structure in rats with heart failure. Neuroscience 424:34–44. https://doi.org/10.1016/j.neuroscience.2019.10.008
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14. https://doi.org/10.1038/nrm3028
Yuan JH, Pan F, Chen J, Chen CE, Xie DP, Jiang XZ, Guo SJ, Zhou J (2017) Neuroprotection by plumbagin involves BDNF-TrkB-PI3K/Akt and ERK1/2/JNK pathways in isoflurane-induced neonatal rats. J Pharm Pharmacol 69(7):896–906. https://doi.org/10.1111/jphp.12681
Zhao Z, Yu Z, Hou Y, Zhang L, Fu A (2020) Improvement of cognitive and motor performance with mitotherapy in aged mice. Int J Biol Sci 16(5):849–858. https://doi.org/10.7150/ijbs.40886
Zhao Y, Zhang J, Zheng Y, Zhang Y, Zhang XJ, Wang H, Du Y, Guan J, Wang X, Fu J (2021) NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J Neuroinflammation 18(1):207. https://doi.org/10.1186/s12974-021-02250-8
Zheng M, Chen M, Liu C, Fan Y, Shi D (2021) Alkaloids extracted from Uncaria rhynchophylla demonstrate neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the PI3K/Akt/mTOR signaling pathway. J Ethnopharmacol 266:113451. https://doi.org/10.1016/j.jep.2020.113451
Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi S, Sreenivasmurthy SG, Liu J, Wang Z, Tong BC, Song J, Lu J, Cheung KH, Li M (2019) Balancing mTOR signaling and autophagy in the treatment of Parkinson's disease. Int J Mol Sci 20(3). https://doi.org/10.3390/ijms20030728
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950. https://doi.org/10.1152/physrev.00026.2013
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82160157, Sheng Wang), the National Natural Science Foundation of China (grant number 81471106, Feng Zhong), and the Guangzhou Basic and Applied Basic Research project of Basic Research plan (grant number 1611, Feng Zhong).
Author information
Authors and Affiliations
Contributions
Yanan Wu and Kaiyi Zhou performed experiments and wrote the manuscript. Baiyang Liu, Jindong Xu, and Liming Lei reviewed the references and completed the experiment. Jiaqi Hu helped with the article modification. Xiao Cheng and Feng Zhong helped with the article modification and designed the experiments. Sheng Wang helped prepare the manuscript and designed the experiments. All authors evaluated the data and read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
The study was approved by the Laboratory Animal Ethics Committee of Guangdong Hospital (reference number: 2021023), and all laboratory animals follow the principles of “the guidelines for Ethical Review of Experimental Animal Welfare” (GB/T35892-2018).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Zhou, K., Liu, B. et al. Glial Activation, Mitochondrial Imbalance, and Akt/mTOR Signaling May Be Potential Mechanisms of Cognitive Impairment in Heart Failure Mice. Neurotox Res 41, 589–603 (2023). https://doi.org/10.1007/s12640-023-00655-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-023-00655-2