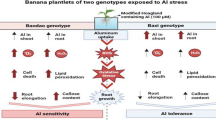
Abstract
Aluminum (Al) is the most plentiful metal present in the Earth’s crust, and when present in acidic soils (pH < 5.0), it becomes soluble and toxic to most plant species. Species from acidic and aluminum-rich soil regions, such as Brazilian Cerrado, developed mechanisms allowing for their growth in these conditions. Some can accumulate Al in their tissues, especially in the leaves. However, the possible functions of Al in these plants are unknown. This study investigated the impact of Al on the organogenesis and production of phenolic compounds in extracts from cotyledonary leaf segments of two Al-accumulator species, Qualea grandiflora Mart. and Vochysia tucanorum Mart. (Vochysiaceae). The addition of Al at 320 μM in the absence of 6-benzylaminopurine (BAP) resulted in the regeneration of both species’ adventitious roots in cotyledonary leaf segments. In contrast, adding BAP without Al did not generate regeneration responses. However, shoot regeneration and development occurred when 0.44 and 0.88 μM BAP was added to the culture medium with 320.0 μM Al. Both species exhibited a noteworthy phenolic content, further enhanced by adding Al and, or, BAP. The antioxidant capacity of extracts also demonstrated a significant increase from the addition of Al and, or, BAP in both species. These findings have important significance for the cultivation and propagation of these species and demonstrate a close relationship between Al and the evolution of these plants. This study is the first to relate Al with phenolic content and antioxidant activity in these two Cerrado plant species, filling a gap in existing research.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Al-Mayahi AMW (2019) Effect of aluminum on the growth of the in vitro culture tissues of the date palm (Phoenix dactylifera L.) cv. Um-Aldehin. Folia Oecol 46:164–169. https://doi.org/10.2478/foecol-2019-0019
Almeida Rodrigues A, Carvalho Vasconcelos Filho S, Müller C, Almeida Rodrigues D, de Fátima SJ, Zuchi J, Carlos Costa A, Lino Rodrigues C, Alves da Silva A, Pereira Barbosa D (2019) Tolerance of Eugenia dysenterica to aluminum: germination and plant growth. Plants 8:317
Amoo SO, Aremu AO, Van Staden J (2012) In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tiss Org Cult 111:345–358
Arruda HS, Araújo MVL, Junior MRM (2022) Underexploited Brazilian Cerrado fruits as sources of phenolic compounds for diseases management: a review. Food Chem Mol Sci:100148
Bojórquez-Quintal E, Escalante-Magaña C, Echevarría-Machado I, Martínez-Estévez M (2017) Aluminum, a friend or foe of higher plants in acid soils. Front Plant Sci 8:1–18. https://doi.org/10.3389/fpls.2017.01767
Bressan ACG, de Oliveira Carvalho Bittencourt BM, Silva GS, Habermann G (2021) Could the absence of aluminum (Al) impair the development of an Al-accumulating woody species from Brazilian savanna? Theor Exp Plant Physiol 33:281–292. https://doi.org/10.1007/s40626-021-00216-y
Brunner I, Sperisen C (2013) Aluminum exclusion and aluminum tolerance in woody plants. Front Plant Sci 4:172
Cardoso JC, da Silva JAT (2013) Micropropagation of Zeyheria montana Mart. (Bignoniaceae), an endangered endemic medicinal species from the Brazilian cerrado biome. In Vitro Cell Dev Biol - Plant 49:710–716. https://doi.org/10.1007/s11627-013-9558-0
Cardoso JC, Silva JAT (2013) Gerbera micropropagation. Biotechnol Adv 31:1344–1357
Casassa LF, Larsen RC, Harbertson JF (2016) Effects of vineyard and winemaking practices impacting berry size on evolution of phenolics during winemaking. Am J Enol Vitic 67:257–268
Chenery EM (1948) Aluminium in the plant world. Kew Bull 3:173. https://doi.org/10.2307/4119757
Colli GR, Vieira CR, Dianese JC (2020) Biodiversity and conservation of the Cerrado: recent advances and old challenges. Biodivers Conserv 29:1465–1475
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tiss Org Cult 106:279–288
Cury NF, Silva RCC, Andre MSF, Fontes W, Ricart CAO, Castro MS, Silveira CES, Williams TCR, de Sousa MV, Pereira LAR (2020) Root proteome and metabolome reveal a high nutritional dependency of aluminium in Qualea grandiflora Mart. (Vochysiaceae). Plant Soil 446:125–143. https://doi.org/10.1007/s11104-019-04323-3
de Andrade LRM, Barros LMG, Echevarria GF, Velho do Amaral LI, Cotta MG, Rossatto DR, Haridasan M, Franco AC (2011) Al-hyperaccumulator Vochysiaceae from the Brazilian Cerrado store aluminum in their chloroplasts without apparent damage. Environ Exp Bot 70:37–42. https://doi.org/10.1016/j.envexpbot.2010.05.013
De Lima ML, Copeland L (1990) The effect of aluminum on the germination of wheat seeds. J Plant Nutr 13:1489–1497. https://doi.org/10.1080/01904169009364170
de Souza MC, Habermann G, do Amaral CL, Rosa AL, MHO P, Da Costa FB (2017) Vochysia tucanorum Mart.: an aluminum-accumulating species evidencing calcifuge behavior. Plant Soil 419:377–389
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Dias WLF, do Vale Junior EP, de Oliveira MDDA, Barbosa YLP, do Nascimento Silva J, da Costa Júnior JS, De Almeida PM, Martins FA (2019) Cytogenotoxic effect, phytochemical screening and antioxidant potential of Jatropha mollissima (Pohl) Baill leaves. South Afr J Bot 123:30–35
Diplock AT (1997) Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease? Free Radic Res 27:511–532
Dörnenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzy Microb Technol 17:674–684
Farias KS, Santos TSN, Paiva M, Almeida SML, Guedes PT, Vianna ACA, Favaro SP, Bueno NR, Castilho RO (2013) Antioxidant properties of species from the Brazilian cerrado by different assays. Rev Bras Plantas Med 15:520–528
Gaba VP (2005) Plant growth regulators in plant tissue culture and development. In: Trigiano RN, Gray DJ (eds) Plant development and biotechnology. CRC Press, Florida, pp 87–100
Guilherme Pereira C, Clode PL, Oliveira RS, Lambers H (2018) Eudicots from severely phosphorus-impoverished environments preferentially allocate phosphorus to their mesophyll. New Phytol 218:959–973. https://doi.org/10.1111/nph.15043
Habermann G, Bressan ACG (2011) Root, shoot and leaf traits of the congeneric Styrax species may explain their distribution patterns in the cerrado sensu lato areas in Brazil. Funct Plant Biol 38:209–218. https://doi.org/10.1071/FP10182
Hajiboland R, Bahrami Rad S, Barceló J, Poschenrieder C (2013) Mechanisms of aluminum-induced growth stimulation in tea (Camellia sinensis). J Plant Nutr Soil Sci 176:616–625
Haridasan M (1982) Aluminium accumulation by some cerrado native species of central Brazil. Plant Soil 65:265–273. https://doi.org/10.1007/BF02374657
Haridasan M (2008) Nutritional adaptations of native plants of the cerrado biome in acid soils. Brazil J Plant Physiol 20:183–195. https://doi.org/10.1590/s1677-04202008000300003
Kang W-Y, Song Y-L, Zhang L (2011) α-Glucosidase inhibitory and antioxidant properties and antidiabetic activity of Hypericum ascyron L. Med Chem Res 20:809–816
Kaur S, Kaur N, Siddique KHM, Nayyar H (2016) Beneficial elements for agricultural crops and their functional relevance in defence against stresses. Arch Agron Soil Sci 62:905–920
Kikui S, Sasaki T, Maekawa M, Miyao A, Hirochika H, Matsumoto H, Yamamoto Y (2005) Physiological and genetic analyses of aluminium tolerance in rice, focusing on root growth during germination. J Inorg Biochem 99:1837–1844. https://doi.org/10.1016/j.jinorgbio.2005.06.031
Klink CA, Machado RB (2005) Conservation of the Brazilian cerrado. Conserv Biol 19:707–713
Kochian LV, Piñeros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66:571–598
Kumar S, Abedin MM, Singh AK, Das S (2020) Role of phenolic compounds in plant-defensive mechanisms. Plant Phenolics Sustain Agric 1:517–532
Kumar S, Korra T, Thakur R, Arutselvan R, Kashyap AS, Nehela Y, Chaplygin V, Minkina T, Keswani C (2023) Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 100154
Leite MS, Pinto TEF, Centofante AR, Neto AR, Silva FG, Selari PJRG, Martins PF (2021) Acclimatization of Pouteria gardeneriana Radlk micropropagated plantlets: role of in vitro rooting and plant growth–promoting bacteria. Curr Plant Biol 27:100209. https://doi.org/10.1016/j.cpb.2021.100209
Liang N, Kitts DD (2014) Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules 19:19180–19208
Lima MR, Gomes HT, Cury NF, Pereira LAR, dos Santos Silveira CE (2022) Developing propagation protocols for Justicia lanstyakii Rizz. (Acanthaceae), an ornamental Ni-accumulating subshrub of Brazilian Cerrado. Biologia (Bratisl) 77:967–980. https://doi.org/10.1007/s11756-021-00987-4
Luczkiewcz M, Cisowski W (2001) Optimisation of the second phase of a two phase growth system for anthocyanin accumulation in callus cultures of Rudbeckia hirta. Plant Cell Tiss Org Cult 65:57–68
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trend Plant Sci 6:273–278. https://doi.org/10.1016/S1360-1385(01)01961-6
Manquian K, Zuniga GE, Barrientos H, Escudey M, Molina M (2013) Effect of aluminum on antioxidant activity and phenolic compounds content in in vitro cultured blueberries. Boletín Latinoam y del Caribe Plantas Med y Aromáticas 12:603–611
Meyer HJ, Van Staden J (1995) The in vitro production of an anthocyanin from callus cultures of Oxalis linearis. Plant Cell Tiss Org Cult 40:55–58
Miura H, Kitamura Y, Ikenaga T, Mizobe K, Shimizu T, Nakamura M, Kato Y, Yamada T, Maitani T, Goda Y (1998) Anthocyanin production of Glehnia littoralis callus cultures. Phytochemistry 48:279–283
Moreira-Araújo RSDR, Barros NVDA, Porto RGCL, Brandão ADCAS, Lima AD, Fett R (2019) Bioactive compounds and antioxidant activity three fruit species from the Brazilian Cerrado. Rev Bras Frutic 41:3–11. https://doi.org/10.1590/0100-29452019011
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nogueira MA, Bressan ACG, Pinheiro MHO, Habermann G (2019) Aluminum-accumulating Vochysiaceae species growing on a calcareous soil in Brazil. Plant Soil 437:313–326. https://doi.org/10.1007/s11104-019-03978-2
Nosko P, Brassard P, Kramer JR, Kershaw KA (1988) The effect of aluminium on seed germination and early seedling establishment, growth, and respiration of white spruce (Picea glauca). Can J Bot 66:2305–2310. https://doi.org/10.1139/b88-313
Oliveira GLDS (2015) Determinação da capacidade antioxidante de produtos naturais in vitro pelo método do DPPH•: estudo de revisão. Rev Bras Plantas Med 17:36–44
Phillips GC, Garda M (2019) Plant tissue culture media and practices: An overview. In Vitro Cell Dev Biol-Plant 55:242–257
Ratter JA, Bridgewater S, Ribeiro JF (2003) Analysis of the floristic composition of the Brazilian cerrado vegetation III: comparison of the woody vegetation of 376 areas. Edinburgh J Bot 60:57–109. https://doi.org/10.1017/s0960428603000064
Rufino M, Alves RE, de Brito ES, de Morais SM, CDG S, Pérez-Jimenez J, Saura-Calixto FD (2007) Metodologia científica: determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Comunicado técnicno online - EMBRAPA
Sarruge JR, Haag HP (1974) Análises químicas em plantas. Piracicaba, ESALQ, p 54
Silambarasan S, Logeswari P, Sivaramakrishnan R, Cornejo P, Sipahutar MK, Pugazhendhi A (2022) Amelioration of aluminum phytotoxicity in Solanum lycopersicum by co-inoculation of plant growth promoting Kosakonia radicincitans strain CABV2 and Streptomyces corchorusii strain CASL5. Sci Total Environ 832:154935
Silva GS, Rodrigues JS, de Oliveira Carvalho BM, Gavassi MA, Bressan ACG, Habermann G (2023) The absence of aluminum compromises the root integrity and reduces leaf hydration and Rubisco performance in Qualea grandiflora, an Al-accumulating species. Plant Biol J 25:740–749. https://doi.org/10.1111/plb.13535
Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculík M (2017) Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot 137:177–193
Tavares DG, Barbosa BVL, Ferreira RL, Duarte WF, Cardoso PG (2018) Antioxidant activity and phenolic compounds of the extract from pigment-producing fungi isolated from Brazilian caves. Biocatal Agric Biotechnol 16:148–154
Tolrà R, Barceló J, Poschenrieder C (2009) Constitutive and aluminium-induced patterns of phenolic compounds in two maize varieties differing in aluminium tolerance. J Inorg Biochem 103:1486–1490
Tosun M, Ercisli S, Sengul M, Ozer H, Polat T, Ozturk E (2009) Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol Res 42:175–181
Tuladhar P, Sasidharan S, Saudagar P (2021) Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In: Jogaiah S (ed) Biocontrol agents and secondary metabolites. Elsevier, pp 419–441
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines (Basel, Switzerland) 5:93. https://doi.org/10.3390/medicines5030093
Vardar F, Arican E, Gozukirmizi N (2006) Effects of aluminum on in vitro root growth and seed germination of tobacco (Nicotiana tabacum L.). Adv Food Sci 28:85–88
Verma V, Zinta G, Kanwar K (2021) Optimization of efficient direct organogenesis protocol for Punica granatum L. cv. Kandhari Kabuli from mature leaf explants. In Vitro Cell Dev Biol-Plant 57:48–59
Vitorello VA, Capaldi FR, Stefanuto VA (2005) Recent advances in aluminum toxicity and resistance in higher plants. Brazil J Plant Physiol 17:129–143. https://doi.org/10.1590/s1677-04202005000100011
Watanabe T, Osaki M (2002) Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: a review. Commun Soil Sci Plant Anal 33:1247–1260
Zhang Y, Cai P, Cheng G, Zhang Y (2022) A brief review of phenolic compounds identified from plants: their extraction, analysis, and biological activity. Nat Prod Commun 17:1934578X211069721
Funding
This study was financially supported by the Brazilian National Council for Scientific and Technological Development (CNPq) (#14056/2019-3).
Author information
Authors and Affiliations
Contributions
MAN, GH, and JCC raised the hypothesis; MAN, VRM, and CJC developed the experimental design; MAN proceeded the experiment and collected the data; MAN, VRM, and JCC wrote the manuscript; VRM measured the phenolic contents and helped to interpret these data; all the authors made significant contributions to the manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
ESM 1
(DOCX 120 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nogueira, M.A., Marin, V.R., Habermann, G. et al. In vitro organogenesis, content phenols, and antioxidant capacity of two aluminum accumulator plant species from the Cerrado region, Brazil. In Vitro Cell.Dev.Biol.-Plant 59, 734–743 (2023). https://doi.org/10.1007/s11627-023-10371-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10371-3