Abstract
Introduction
Although one of the major presentations of vestibular migraine is dizziness with/without unsteady gait, it is still classified as one of the migraine categories. However, in contrast to ordinary migraine, vestibular migraine patients have distinct characteristics, and the detailed treatment strategy for vestibular migraine is different and more challenging than ordinary migraine treatment. Currently, there is no conclusive evidence regarding its management, including vestibular migraine prophylaxis.
Aim
The objective of this current network meta-analysis (NMA) was to compare the efficacy and acceptability of individual treatment strategies in patients with vestibular migraine.
Methods
The PubMed, Embase, ScienceDirect, ProQuest, Web of Science, ClinicalKey, Cochrane Central, and ClinicalTrials.gov databases were systematically searched for randomized controlled trials (RCTs), with a final literature search date of 30 December 2022. Patients diagnosed with vestibular migraine were included. The PICO of the current study included (1) patients with vestibular migraine; (2) intervention: any active pharmacologic or non-pharmacologic intervention; (3) comparator: placebo-control, active control, or waiting list; and (4) outcome: changes in migraine frequency or severity. This NMA of RCTs of vestibular migraine treatment was conducted using a frequentist model. We arranged inconsistency and similarity tests to re-examine the assumption of NMA, and also conducted a subgroup analysis focusing on RCTs of pharmacological treatment for vestibular migraine management. The primary outcome was changes in the frequency of vestibular migraines, while the secondary outcomes were changes in vestibular migraine severity and acceptability. Acceptability was set as the dropout rate, which was defined as the participant leaving the study before the end of the trial for any reason. Two authors independently evaluated the risk of bias for each domain using the Cochrane risk-of-bias tool.
Results
Seven randomized controlled trials (N = 828, mean age 37.6 years, 78.4% female) and seven active regimens were included. We determined that only valproic acid (standardized mean difference [SMD] −1.61, 95% confidence interval [CI] −2.69, −0.54), propranolol (SMD −1.36, 95% CI −2.55, −0.17), and venlafaxine (SMD −1.25, 95% CI −2.32, −0.18) were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups. Furthermore, among all the investigated pharmacologic/non-pharmacologic treatments, valproic acid yielded the greatest decrease in vestibular migraine frequency among all the interventions. In addition, most pharmacologic/non-pharmacologic treatments were associated with similar acceptability (i.e. dropout rate) as those of the placebo/control groups.
Conclusions
The current study provides evidence that only valproic acid, propranolol, and venlafaxine might be associated with beneficial efficacy in vestibular migraine treatment.
Trial registration
CRD42023388343.
Similar content being viewed by others
Vestibular migraines have long been ignored and misdiagnosed. In contrast to ordinary migraine, vestibular migraine patients have distinct characteristics, and the detailed treatment strategy for vestibular migraine is different and more challenging than ordinary migraine treatment. Currently, there is no conclusive evidence regarding its management, including vestibular migraine prophylaxis. |
This network meta-analysis of seven randomized controlled trials (RCTs) of vestibular migraine treatment demonstrated that only valproic acid, propranolol, and venlafaxine were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups. Furthermore, among all the investigated pharmacologic/non-pharmacologic treatments, valproic acid yielded the most decreased vestibular migraine frequency among all the interventions. In addition, most pharmacologic/non-pharmacologic treatments were associated with acceptability and safety profiles similar to those of the placebo/control groups. |
The current study provides evidence that only valproic acid, propranolol, and venlafaxine might be associated with beneficial efficacy in vestibular migraine treatment. |
1 Introduction
Despite the symptomatology of dizziness during a migraine attack, vestibular migraine is considered a distinct subtype of migraine [1,2,3]. Vestibular symptoms can occur in the absence of headache or migraine episodes in patients with vestibular migraine. Unlike ordinary migraine, vestibular migraine has distinct characteristics [4]. Specifically, patients with vestibular migraine had a significantly higher balance disability but fewer headache symptoms than patients with migraine. Furthermore, vestibular migraine patients are more likely to be depressed than normal migraine patients [4]. The management strategy for vestibular migraine is similar to that for ordinary migraine. Specifically, the management strategy for vestibular migraine would emphasize acute treatment to an acute attack and prophylactic treatment to prevent the next episode. However, the detailed treatment strategy for vestibular migraine is different and more challenging than ordinary migraine treatment [5, 6].
Several large-scale network meta-analyses (NMAs) of pharmacologic [7, 8] or non-pharmacologic [9] treatments have focused on ordinary migraine prophylaxis; however, unlike ordinary migraines, there have been few studies on vestibular migraine prophylaxis. Most of these studies had low evidence levels, such as retrospective reports or non-RCTs [5]. Sharing the overlapping pathophysiology with ordinary migraine, some effective medications in ordinary migraine prophylaxis, such as valproic acid, also provide efficacy in vestibular migraine prophylaxis [10]. However, not all medications that are effective for ordinary migraine have significant efficacy for vestibular migraine. For example, a recent RCT failed to show the efficacy of metoprolol in vestibular management [11]. Among the currently available trials, most did not clearly distinguish participants with vestibular migraine from those with ordinary migraine. Therefore, these methodological limitations would restrict our interpretation and application of these results in clinical practice for patients with vestibular migraine [5].
The NMA has the advantage of allowing for multiple comparisons of efficacy between individual treatment strategies for vestibular migraine prophylaxis. Such evidence from an NMA can inform clinical practice [12]. The aim of our NMA was to compare the efficacy and acceptability of individual treatment strategies in patients with vestibular migraine. In addition, to provide evidence that is more specific to vestibular migraine, we only included RCTs of definite vestibular migraine, which were diagnosed according to specific well-defined diagnostic guidelines [1,2,3].
2 Methods
2.1 General Description for the Current Study
The current NMA complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [13] and AMSTAR 2 guidelines [14]. After approval by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center (TSGHIRB No. B-109-29), we registered this study in the PROSPERO database (registration: CRD42023388343).
2.2 Searches
We retrieved eligible RCTs from the PubMed, Embase, ScienceDirect, ProQuest, Web of Science, ClinicalKey, Cochrane CENTRAL, and ClinicalTrials.gov databases (electronic supplementary material [ESM] Table 1); the gray literature was searched in ClinicalTrials.gov. The final date of the literature search was 30 December 2022. No language restrictions were applied. We also arranged a manual search to find articles cited in review articles and pairwise meta-analyses [15,16,17,18].
2.3 Inclusion/Exclusion Criteria
The PICO of the current study included (1) patients with vestibular migraine; (2) intervention: any active pharmacologic or non-pharmacologic intervention; (3) comparator: placebo-control, active control, or waiting list; and (4) outcome: changes in migraine frequency or severity. We only included RCTs that reported on vestibular migraine patients for efficacy of pharmacological or non-pharmacological interventions for vestibular migraine management. The inclusion criteria were (1) RCTs; (2) human participants; and (3) patients with a diagnosis of vestibular migraine. A diagnosis of vestibular migraine can be established according to well-defined diagnostic guidelines [1,2,3]. Exclusion criteria were (1) not clinical trials; (2) not RCTs; (3) did not include target outcomes of interest; or (4) not specific to patients with vestibular migraine. In situations where the same set of data had been used by multiple studies, we used the study with the most information or with the largest sample size.
2.4 Data Extraction
The eligible studies were screened by two authors to determine their inclusion/exclusion. Data of interest were extracted from the included studies, and the risk of bias was assessed by the aforementioned two authors. Where these authors disagreed, the corresponding author adjudicated the disagreements. If the manuscript lacked relevant data, we contacted the corresponding authors or co-authors to obtain the data of interest. We followed the research process of our previous NMAs on migraine management [7,8,9, 19,20,21].
2.5 Variables and Outcomes
Because the goal of treatment for vestibular migraine is not complete remission but a reduction in vestibular migraine frequency [22, 23], we selected the changes in vestibular migraine frequency to be the primary outcome. With regard to data extraction of the outcome ‘changes in migraine frequency’, because not all vestibular migraineur patients were able to clearly recognize the current episode to be a vestibular migraine attack, other type of headache, or other type of vertigo, the RCTs applying migraine diaries might have some methodological limitations. Therefore, if there was ‘changes in migraine frequency’, ‘changes in headache frequency’, and ‘changes in vertigo frequency’ in one RCT, we selected ‘changes in migraine frequency’ first. If there were no ‘changes in migraine frequency’, we chose to use ‘changes in headache frequency’ or ‘changes in vertigo frequency’. The secondary outcomes were changes in the vestibular migraine severity, acceptability, and safety profiles. Acceptability was calculated using the dropout rate, i.e., the proportion of patients leaving the study before the end of the trial due to any reason. The safety profile was set as any adverse effect rate, which was defined as a participant encountering any adverse effect during the study regardless of whether the treatment was related or not.
2.6 Cochrane Risk-of-Bias Tool
The risk of bias (interrater reliability = 0.85) was assessed by two authors using the Cochrane risk-of-bias tool [24].
2.7 Statistical Analysis
We performed NMA using STATA version 16.0 (StataCorp LLC, College Station, TX, USA). The standardized mean difference (SMD) and odds ratio (OR), accompanied with their corresponding 95% confidence intervals (CIs), were calculated for continuous and categorical data, respectively. With regard to OR, we applied a 0.5 zero-cell correction in the meta-analysis procedure; however, for studies with 0 in both the intervention and control arms, we did not apply such a correction because bias might be increased by doing so [25, 26]. The current NMA was based on the frequentist model. A two-tailed test, with a p value <0.05 indicating statistical significance, was used to compare the effect size. Heterogeneity was evaluated using the tau statistic.
We arranged a mixed comparison with a generalized linear mixed model to analyze the direct/indirect comparisons in this NMA [27]. The indirect comparisons were conducted by the assumption of transitivity; in other words, we assumed that the hitherto unknown difference between treatments A and B could be determined from the known differences between A and C and between B and C, where C is the third treatment. Furthermore, to make multi-arm comparisons, we combined direct/indirect evidences from the recruited studies [28]. We used the mvmeta command in STATA [29]. The restricted maximum likelihood method was applied to investigate the between-study variance [30]. To increase the clinical applicability of our findings, we calculated the surface under the cumulative ranking curve (SUCRA) to rank the probability of relative superiority of individual treatments compared with others [31]. Although the concept of probability was mainly based on the Bayesian model, the module of White (2015) could simulate the probability and deal with a multi-arm trial according to the calculated data from the frequentist model so that we could achieve the SUCRA conclusion [32].
Finally, to support the assumptions of similarity/transitivity/consistency, we arranged the following analyses. First, in line with the rationale of our previous NMA studies [9, 33], this study assessed the effectiveness of the different control interventions (i.e., changes in vestibular migraine frequency by either controls or placebo) to justify our assumption of similarity. Specifically, this study computed the changes in vestibular migraine frequency in the ‘RCT with placebo’ group and ‘RCT with controls’ group using Comprehensive Meta-Analysis (version 3; Biostat, Englewood, NJ, USA) [34]. Later, we evaluated transitivity/consistency using a loop-specific approach, node-splitting method, and design-by-treatment model [35].
The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tools were chosen to evaluate the quality of overall evidence following the rationale of previous studies conducted by Puhan et al. [36] and Cipriani et al. [37]. Finally, to reduce the potential source of heterogeneity, we conducted a subgroup analysis focusing on RCTs of pharmacological treatment for vestibular migraine management.
3 Results
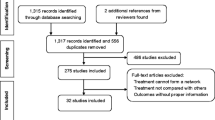
After the initial screening procedure, 23 articles were considered for full-text review (Fig. 1). Overall, 16 articles were excluded for various reasons (Fig. 1 and ESM Table 2) [15,16,17,18, 38,39,40,41,42,43,44,45,46,47,48,49], leaving seven articles for final inclusion in the NMA (Table 1) [11, 50,51,52,53,54,55]. The overall network structure of the treatment arms is shown in Fig. 2.
Overall network structure of the current network meta-analysis for the primary outcome of changes in vestibular migraine frequency. The lines between nodes represent direct comparisons in various trials, and the size of each circle is proportional to the number of participants receiving each specific treatment. The thickness of the lines is proportional to the number of trials connected to the network. Flu flunarizine, LcS probiotic of L. casei Shirota, Met metoprolol, PlaTAU placebo or treatment as usual, Pro propranolol, Res resistance exercise, Val valproic acid, Ven venlafaxine
3.1 Characteristics
These seven RCTs, published between 2014 and 2022, included 828 participants in total. The mean age was 37.6 years (range 32.5–52.4 years) and 78.4% of participants were female (range 60.8–92.2%). The mean study duration was 14.9 weeks (range 12–24 weeks). None of the RCTs prohibited the concurrent use of anti-migraine medication. The vestibular migraine diagnosis was made according to Neuhauser et al. [1], Lempert et al. [2], or the International Classification of Headache Disorders 3rd edition criteria [3]. The investigated treatment arms included seven active regimens (flunarizine, propranolol, venlafaxine, metoprolol, valproic acid, resistance exercise, and probiotic L. casei Shirota) and one control arm (placebo or treatment as usual).
3.2 Primary Outcome: The Changes in Vestibular Migraine Frequency
The main results revealed that valproic acid (SMD −1.61, 95% CI −2.69, −0.54), propranolol (SMD −1.36, 95% CI −2.55, −0.17), and venlafaxine (SMD −1.25, 95% CI −2.32, −0.18) were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups (Table 2, Figs. 2, 3). With regard to clinical applicability, we calculated the probability of relative superiority of individual treatments compared with others according to the SUCRA analysis. To be specific, valproic acid yielded the most decreased vestibular migraine frequency among all interventions (ESM Table 3A).
Forest plot of the primary outcome—changes in vestibular migraine frequency. When the effect size was <0 (presented as the SMD), the specified treatment yielded a better improvement in vestibular migraine frequency than the control group. CI confidence interval, Flu flunarizine, LcS probiotic of L. casei Shirota, Met metoprolol, PlaTAU placebo or treatment as usual, Pro propranolol, Res resistance exercise, SMD standardized mean difference, Val valproic acid, Ven venlafaxine
Subgroup analysis based on RCTs of pharmacological treatment revealed similar findings. Specifically, valproic acid (SMD −1.61, 95% CI −2.73, −0.50), propranolol (SMD −1.36, 95% CI −2.60, −0.12), and venlafaxine (SMD −1.25, 95% CI −2.36, −0.14) were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups (ESM Table 4A, ESM Fig. 1A, and ESM Fig. 2B). Similarly, in consideration of the clinically relative superiority of efficacy, valproic acid yielded the most decreased vestibular migraine frequency among all interventions according to the SUCRA results (ESM Table 3B).
The test of similarity revealed that there was no significant difference in the changes of vestibular migraine frequency between the ‘RCT with placebo’ and ‘RCT with controls’ groups (p = 0.101) (ESM Fig. 2A).
3.3 Secondary Outcome: Changes of Vestibular Migraine Severity
The main result of the NMA revealed that none of the investigated treatments were associated with significantly different improvements in vestibular migraine severity compared with the placebo/control groups (ESM Table 4B, ESM Table 3C, ESM Fig. 1B, and ESM Fig. 2C).
3.4 Acceptability with Respect to the Dropout Rate
The main result of the NMA revealed that none of the investigated treatments were associated with significantly different dropout rates compared with the placebo/control groups (ESM Table 4C, ESM Table 3D, ESM Fig. 1C, and ESM Fig. 2D).
3.5 Safety Profile with Respect to Any Adverse Effect Rate
The main result of the NMA revealed that none of the investigated treatments were associated with significantly different adverse effect rates compared with the placebo/control groups (ESM Table 4D, ESM Table 3E, ESM Fig. 1D, and ESM Fig. 2E).
3.6 Risk of Bias and Publication Bias
We found that 75.5% (37/49 items), 8.2% (4/49 items), and 16.3% (8/49 items) of the included studies had low, unclear, and high risks of bias, respectively. The vague reporting of allocation concealment and the blindness of the participants/investigator contributed to the risk of bias (ESM Fig. 3A, B).
Funnel plots of publication bias across the included studies (ESM Figs. 4A–H) revealed general symmetry, and the results of the Egger’s test indicated no significant publication bias among the articles included in the NMA. The aforementioned test of similarity revealed insignificant differences (p = 0.101) (ESM Fig. 2A). Furthermore, in general, the current NMA does not exhibit inconsistency, whether local inconsistency (assessed using the loop-specific approach and node-splitting method) or global inconsistency (assessed using the design-by-treatment method), therefore there was no evidence to refute the assumption of similarity/transitivity/consistency (ESM Table 5, ESM Table 6). No significant heterogeneity was detected by tau value (ESM Table 7). The GRADE rating revealed that the quality of evidence for most comparisons in the current NMA ranged from low to medium (ESM Table 8).
4 Discussion
The main finding of the present NMA is that most brain-acting regimens (i.e., valproic acid, propranolol, and venlafaxine) were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups. Furthermore, among all the investigated pharmacologic/non-pharmacologic treatments, valproic acid yielded the most decreased vestibular migraine frequency among all the interventions. In addition, most pharmacologic/non-pharmacologic treatments were associated with acceptability and safety profiles similar to those of the placebo/control groups.
Our NMA observed that most brain-acting regimens (i.e., valproic acid [51], propranolol [53], and venlafaxine [51, 53]) were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups. Valproic acid was associated with the most decreased vestibular migraine frequency among all interventions. This finding was consistent with our previous large-scale NMA regarding ordinary migraine [8], in which we observed that high-dose melatonin, valproic acid, and topiramate were all associated with significant improvement in ordinary migraine frequency in comparison with placebo controls. There have been arguments regarding the different treatment efficacies of individual pharmacological treatments for ordinary migraine and vestibular migraine. Considering the main findings of these two NMAs, we noted similar and different parts. Specifically, the anticonvulsant valproic acid was effective against both vestibular migraine and migraine. This beneficial effect could be derived from the physiopathology of vestibular migraine that the asymmetrical activation of the vestibular nucleus, which is mediated by balanced glutamate and gamma-aminobutyric acid (GABA), is associated with vertigo symptoms in vestibular migraine [56]. Therefore, the prescription of valproic acid, which would inhibit N-methyl-D-aspartate receptors and modulate intra-brain GABA levels, would contribute to its efficacy in vestibular migraine management [10].
Another important finding of the current NMA was that the other two brain-acting regimens (i.e., propranolol [53] and venlafaxine [51]) were both significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups. Venlafaxine, a regimen with serotonergic effects, was only effective in vestibular migraine but not in ordinary migraine [8]. These different findings might be supported by a previous cross-sectional analytic study that vestibular migraine patients are significantly more anxious and agoraphobic than ordinary migraine patients at baseline [57]. Similarly, in another important trial, the prescription of serotonergic regimens in patients with dizziness, either with an otogenic or pure psychogenic etiology, resulted in significant improvement in both anxiety and dizziness severity [58]. Therefore, theoretically, a venlafaxine prescription would be effective for vestibular management. In contrast, propranolol, classified as a competitive non-cardioselective sympatholytic β-blocker, was only effective in vestibular migraine and not in ordinary migraine [8]. Propranolol, which can cross the blood–brain barrier, acts not only on the β-adrenergic receptor but also weakly on certain serotonin receptors, such as 5-HT1A, 5-HT1B, and 5-HT2B [59], thus exerting anxiolytic effects [59]. According to a previous clinical trial, uncontrolled comorbid anxiety is a risk factor for reduced treatment response to vestibular migraine [60]. Considering the above evidence together, we hypothesized that the beneficial effects of venlafaxine and propranolol on vestibular migraine management might be derived from reduced comorbid anxiety. However, since the overall included RCTs were relatively few and the evidences of the efficacy of valproate, propranolol, and venlafaxine versus the placebo/control group mainly came from an indirect comparison, the results of the current NMA are preliminary. Furthermore, the evidence from an indirect comparison would be weaker than those from direct evidence, therefore the power of an indirect comparison would be relatively small. Therefore, we may need future large-scale RCTs to form a direct comparison between the aforementioned treatments so that we might achieve more conclusive evidence [61, 62]. In addition, although no significant heterogeneity was detected via statistical examination or in the overall demographic data of the subjects within the recruited RCTs, some analyses in our NMA might be limited by potential heterogeneity between studies. With regard to the treatment efficacy of resistance exercise [54], probiotics [52], metoprolol [11], and flunarizine [50, 55], the statistical insignificance might have resulted from the low sample size and low power due to the indirect comparison. Finally, although some RCTs of cinnarizine, clonazepam, topiramate, amitriptyline, triptans, or monoclonal antibodies in ordinary migraine management have been conducted, we did not include the aforementioned treatments in this NMA due to a lack of RCTs of those regimens in vestibular migraine management.
Our NMA had some limitations that need to be addressed. First, some analyses in our NMA were limited by potential heterogeneity between studies with respect to participant characteristics such as underlying diseases, concomitant medication, age, heterogeneous diagnostic criteria, and trial duration. Second, some of the included studies had small sample sizes, which may have resulted in less robust quantitative findings. Third, some of the included RCTs did not apply placebo controls, and a placebo effect could have affected their findings. Fourth, as previously mentioned, we did not include the treatment of cinnarizine, metoprolol, clonazepam, topiramate, amitriptyline, triptans, or monoclonal antibodies due to the lack of RCTs of these regimens in vestibular migraine management. Finally, most RCTs in the current NMA had relatively short study durations (mean follow-up duration 14.9 weeks). Further studies with longer study periods are required.
5 Conclusion
The present NMA demonstrated that most brain-acting regimens (i.e., valproic acid, propranolol, and venlafaxine) were significantly associated with better improvement in vestibular migraine frequency than the placebo/control groups. Furthermore, among all the investigated pharmacologic/non-pharmacologic treatments, valproic acid was associated with the most decreased vestibular migraine frequency among all interventions. In addition, most pharmacologic/non-pharmacologic treatments were associated with acceptability and safety profiles similar to those of the placebo/control groups. Because of the limitations of the small sample sizes, our findings imply the need for future large-scale RCTs to support or refute the findings of the current NMA.
References
Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56(4):436–41.
Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167–72.
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Ozcelik P, Kocoglu K, Ozturk V, Keskinoglu P, Akdal G. Characteristic differences between vestibular migraine and migraine only patients. J Neurol. 2022;269(1):336–41.
Bisdorff AR. Management of vestibular migraine. Ther Adv Neurol Disord. 2011;4(3):183–91.
Smyth D, Britton Z, Murdin L, Arshad Q, Kaski D. Vestibular migraine treatment: a comprehensive practical review. Brain. 2022;145(11):3741–54.
Yang CP, Zeng BY, Chang CM, Shih PH, Yang CC, Tseng PT, et al. Comparative effectiveness and tolerability of the pharmacology of monoclonal antibodies targeting the calcitonin gene-related peptide and its receptor for the prevention of chronic migraine: a network meta-analysis of randomized controlled trials. Neurotherapeutics. 2021;18(4):2639–50.
Tseng PT, Yang CP, Su KP, Chen TY, Wu YC, Tu YK, et al. The association between melatonin and episodic migraine: a pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J Pineal Res. 2020;69(2): e12663.
Cheng YC, Zeng BY, Hung CM, Su KP, Wu YC, Tu YK, et al. Effectiveness and acceptability of noninvasive brain and nerve stimulation techniques for migraine prophylaxis: a network meta-analysis of randomized controlled trials. J Headache Pain. 2022;23(1):28.
Celiker A, Bir LS, Ardic N. Effects of valproate on vestibular symptoms and electronystagmographic findings in migraine patients. Clin Neuropharmacol. 2007;30(4):213–7.
Bayer O, Adrion C, Al Tawil A, Mansmann U, Strupp M, Investigators P. Results and lessons learnt from a randomized controlled trial: prophylactic treatment of vestibular migraine with metoprolol (PROVEMIG). Trials. 2019;20(1):813.
Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628–30.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008.
Chen J, Zhao W, Yue X, Zhang P. Risk factors for the occurrence of benign paroxysmal positional vertigo: a systematic review and meta-analysis. Front Neurol. 2020;11:506.
Espinosa-Sanchez JM, Lopez-Escamez JA. The pharmacological management of vertigo in Meniere disease. Expert Opin Pharmacother. 2020;21(14):1753–63.
Strupp M, Versino M, Brandt T. Vestibular migraine. Handb Clin Neurol. 2010;97:755–71.
Togha M, Martami F, Abdollahi M, Mozafari M, Cheraghali H, Rafiee P, et al. Cinnarizine as an alternative recommendation for migraine prophylaxis: a narrative review. Expert Rev Neurother. 2020;20(9):943–51.
Tseng PT, Zeng BY, Zeng BS, Liao YC, Stubbs B, Kuo JS, et al. Omega-3 polyunsaturated fatty acids in sarcopenia management: a network meta-analysis of randomized controlled trials. Ageing Res Rev. 2023;90: 102014.
Tseng PT, Chiu HJ, Suen MW, Zeng BS, Wu MK, Tu YK, et al. Pharmacological interventions and hormonal therapies for depressive symptoms in peri- and post-menopausal women: a network meta-analysis of randomized controlled trials. Psychiatry Res. 2023;326: 115316.
Tseng PT, Zeng BS, Suen MW, Wu YC, Correll CU, Zeng BY, et al. Efficacy and acceptability of anti-inflammatory eicosapentaenoic acid for cognitive function in Alzheimer’s dementia: a network meta-analysis of randomized, placebo-controlled trials with omega-3 fatty acids and FDA-approved pharmacotherapy. Brain Behav Immun. 2023;111:352–64.
Kalita J, Laskar S, Bhoi SK, Misra UK. Efficacy of single versus three sessions of high rate repetitive transcranial magnetic stimulation in chronic migraine and tension-type headache. J Neurol. 2016;263(11):2238–46.
Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(Suppl 2):103–22 (quiz 23–6).
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009.
Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6(8): e010983.
Brockhaus AC, Bender R, Skipka G. The Peto odds ratio viewed as a new effect measure. Stat Med. 2014;33(28):4861–74.
Tu YK. Use of generalized linear mixed models for network meta-analysis. Med Decision Making. 2014;34(7):911–8.
Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–24.
White IR. Network meta-analysis. Stata J. 2015;15(4):951–85.
Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS ONE. 2013;8(7): e69930.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71.
White IR. Network meta-analysis. Stata J. 2015;15:951–85.
Tseng PT, Zeng BS, Hung CM, Liang CS, Stubbs B, Carvalho AF, et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: a systematic review and network meta-analysis. JAMA Psychiat. 2022;79(8):770–9.
Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219.
Higgins JP, Del Giovane C, Chaimani A, Caldwell DM, Salanti G. Evaluating the quality of evidence from a network meta-analysis. Value Health. 2014;17(7):A324.
Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349: g5630.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–66.
Carvalho GF, Mehnert J, Basedau H, Luedtke K, May A. Brain processing of visual self-motion stimuli in patients with migraine: an fMRI study. Neurology. 2021;97(10):e996–1006.
Clemow DB, Baygani SK, Hauck PM, Hultman CB. Lasmiditan in patients with common migraine comorbidities: a post hoc efficacy and safety analysis of two phase 3 randomized clinical trials. Curr Med Res Opin. 2020;36(11):1791–806.
Du Y, Liu X, Ren L, Wang Y, Wu Z. Analysis of video head impulse test saccades data in patients with vestibular migraine or probable vestibular migraine. J Otol. 2022;17(4):197–202.
Filippopulos FM, Strobl R, Belanovic B, Dunker K, Grill E, Brandt T, et al. Validation of a comprehensive diagnostic algorithm for patients with acute vertigo and dizziness. Eur J Neurol. 2022;29(10):3092–101.
Gode S, Celebisoy N, Kirazli T, Akyuz A, Bilgen C, Karapolat H, et al. Clinical assessment of topiramate therapy in patients with migrainous vertigo. Headache. 2010;50(1):77–84.
Gorur K, Gur H, Ismi O, Ozcan C, Vayisoglu Y. The effectiveness of propranolol, flunarizine, amitriptyline and botulinum toxin in vestibular migraine complaints and prophylaxis: a non-randomized controlled study. Braz J Otorhinolaryngol. 2022;88(6):975–81.
Hu T, Hu H, Chen F, Jiang B, Shen F, Su Y, et al. The efficacy and safety of acupuncture for prophylaxis of vestibular migraine: a study protocol for a randomized controlled trial. Front Neurol. 2021;12: 709803.
Koc A, Cevizci AE. Effects of vestibular rehabilitation in the management of patients with and without vestibular migraine. Braz J Otorhinolaryngol. 2022;88(Suppl 3):S25–33.
Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology. 2003;60(5):882–3.
Strupp M, Dlugaiczyk J, Ertl-Wagner BB, Rujescu D, Westhofen M, Dieterich M. Vestibular disorders. Dtsch Arztebl Int. 2020;117(17):300–10.
Tassorelli C, Bragg S, Krege JH, Doty EG, Ardayfio PA, Ruff D, et al. Safety findings from CENTURION, a phase 3 consistency study of lasmiditan for the acute treatment of migraine. J Headache Pain. 2021;22(1):132.
Wilkinson D, Ade KK, Rogers LL, Attix DK, Kuchibhatla M, Slade MD, et al. Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache. 2017;57(7):1065–87.
Lepcha A, Amalanathan S, Augustine AM, Tyagi AK, Balraj A. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol. 2014;271(11):2931–6.
Liu F, Ma T, Che X, Wang Q, Yu S. The efficacy of venlafaxine, flunarizine, and valproic acid in the prophylaxis of vestibular migraine. Front Neurol. 2017;8:524.
Qi X, Fan G, Jia H. The probiotic Lactobacillus casei Shirota attenuates symptoms of vestibular migraine: a randomised placebo-controlled double-blind clinical trial. Benef Microbes. 2020;11(5):469–76.
Salviz M, Yuce T, Acar H, Karatas A, Acikalin RM. Propranolol and venlafaxine for vestibular migraine prophylaxis: a randomized controlled trial. Laryngoscope. 2016;126(1):169–74.
Sun L, Li G, Liu F, Wang Y, Zhang L, Minoret C. Resistance exercise relieves symptoms of vestibular migraine patients with MRI diagnosis: a randomized parallel-controlled single-blind clinical trial. Revue neurologique. 2022;178(4):370–6.
Yuan Q, Liu DL, Yu LS, Zhang QF. Flunarizine in the prophylaxis of vestibular migraine: a randomized controlled trial. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30(10):805–10.
Smith PF. Pharmacology of the vestibular system. Curr Opin Neurol. 2000;13(1):31–7.
Kutay O, Akdal G, Keskinoglu P, Balci BD, Alkin T. Vestibular migraine patients are more anxious than migraine patients without vestibular symptoms. J Neurol. 2017;264(Suppl 1):37–41.
Staab JP, Ruckenstein MJ. Chronic dizziness and anxiety: effect of course of illness on treatment outcome. Arch Otolaryngol Head Neck Surg. 2005;131(8):675–9.
Szeleszczuk L, Fraczkowski D. Propranolol versus other selected drugs in the treatment of various types of anxiety or stress, with particular reference to stage fright and post-traumatic stress disorder. Int J Mol Sci. 2022;23(17):10099.
Kisabay Ak A, Celebisoy N, Ozdemir HN, Gokcay F, Saruhan Durmaz G, Top Karti D, et al. Factors determining the response to treatment in patients with vestibular migraine. Neurol Res. 2022;44(9):847–54.
Thorlund K, Mills EJ. Sample size and power considerations in network meta-analysis. Syst Rev. 2012;1:41.
Mills EJ, Ghement I, O’Regan C, Thorlund K. Estimating the power of indirect comparisons: a simulation study. PLoS ONE. 2011;6(1): e16237.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No research funding was received for this work.
Conflicts of interest/competing interests
Jiann-Jy Chen, Bing-Syuan Zeng, Kuan-Pin Su, Yi-Cheng Wu, Yu-Kang Tu, Brendon Stubbs, Tien-Yu Chen, Bing-Yan Zeng, Yen-Wen Chen, Chih-Wei Hsu, Ping-Tao Tseng declare that they have no any potential conflicts of interest in relation to this work.
Ethics approval
The current study complied with the Declaration of Helsinki, and was approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center (TSGHIRB No. B-109-29).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data in the current study are available upon reasonable request. CWH and PTT had full access to all the data in the study, conducted the data analysis, and take full responsibility for the integrity of the data and the data analysis, and the accuracy of the data analysis.
Code availability
The code from the current study is available upon reasonable request.
Author contributions
JJC and BSZ contributed equally as first authors and were responsible for the literature search, data extraction, and manuscript draft. KPS, YCW, YKT, BS, TYC, BYZ, and YWC were responsible for the study design, concept formation, and major revision of the manuscript. CWH and PTT contributed equally as corresponding authors and were responsible for the data correction, manuscript revision, and manuscript submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, JJ., Zeng, BS., Su, KP. et al. Network Meta-analysis of Different Treatments for Vestibular Migraine. CNS Drugs 37, 837–847 (2023). https://doi.org/10.1007/s40263-023-01037-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01037-0