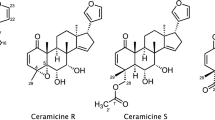
Abstract
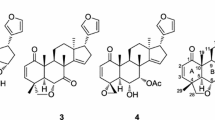
Ceramicines are a series of limonoids which were isolated from the barks of Malaysian Chisocheton ceramicus (Meliaceae), and were known to show various biological activity. Six new limonoids, ceramicines U–Z (1–6), with a cyclopentanone[α]phenanthrene ring system with a β-furyl ring at C-17 were isolated from the barks of C. ceramicus. Their structures were determined on the basis of the 1D and 2D NMR analyses, and their absolute configurations were investigated by CD spectroscopy. Ceramicine W (3) exhibited potent antimalarial activity against Plasmodium falciparum 3D7 strain with IC50 value of 1.2 µM. In addition, the structure–antimalarial activity relationship (SAR) of the ceramicines was investigated to identify substituent patterns that may enhance activity. It appears that ring B and the functional groups in the vicinity of rings B and C are critical for the antimalarial activity of the ceramicines. In particular, bulky ester substituents with equatorial orientation at C-7 and C-12 greatly increase the antimalarial activity.
Graphical Abstract





Similar content being viewed by others
References
Wiesner J, Ortmann R, Jomaa H, Schlitzer M (2003) New antimalarial drugs. Angew Chem 42:5274–5293
Nugroho AE, Ono Y, Jin E, Hirasawa Y, Kaneda T, Rahman A, Kusumawati I, Tougan T, Horii T, Zaini NC, Morita H (2021) Bisindole alkaloids from Voacanga grandifolia leaves. J Nat Med 75:408–414
Amelia P, Nugroho AE, Hirasawa Y, Kaneda T, Tougan T, Horii T, Morita H (2021) Two new bisindole alkaloids from Tabernaemontana macrocarpa Jack. J Nat Med 75:633–642
Hirasawa Y, Yasuda R, Minami W, Hirata M, Nugroho AE, Tougan T, Uchiyama N, Hakamatsuka T, Horii T, Morita H (2021) Divaricamine A, a new anti-malarial trimeric monoterpenoid indole alkaloid from Tabernaemontana divaricata. Tetrahedron Lett 83:153423
Tang Y, Nugroho AE, Hirasawa Y, Tougan T, Horii T, Hadi AHA, Morita H (2019) Leucophyllinines A and B, bisindole alkaloids from Leuconotis eugeniifolia. J Nat Med 73:533–540
Hirasawa Y, Dai X, Deguchi J, Hatano S, Sasaki T, Ohtsuka R, Nugroho AE, Kaneda T, Morita H (2019) New vasorelaxant indole alkaloids, taberniacins A and B, from Tabernaemontana divaricata. J Nat Med 73:627–632
Amelia P, Nugroho AE, Hirasawa Y, Kaneda T, Tougan T, Horii T, Morita H (2019) Two new sarpagine-type indole alkaloids and antimalarial activity of 16-demethoxycarbonylvoacamine from Tabernaemontana macrocarpa Jack. J Nat Med 73:820–825
Nugroho AE, Zhang W, Hirasawa Y, Tang Y, Wong CP, Kaneda T, Hadi AHA, Morita H (2018) Bisleuconothines B-D, modified eburnane-aspidosperma bisindole alkaloids from Leuconotis griffithii. J Nat Prod 81:2600–2604
Prema WCP, Kodama T, Nugroho AE, El-Desoky AH, Awouafack MD, Win YY, Ngwe H, Abe I, Morita H, Morita H (2020) Three new quassinoids isolated from the wood of Picrasma javanica and their anti-Vpr activities. J Nat Med 74:571
Prema WCP, Nugroho AE, Awouafack MD, Win YY, Win NN, Ngwe H, Morita H, Morita H (2019) Two new quassinoids and other constituents from Picrasma javanica wood, and their biological activities. J Nat Med 73:589–596
Nugroho AE, Wong CP, Hirasawa Y, Kaneda T, Tougan T, Horii T, Hadi AHA, Morita H (2023) Antimalarial ceramicines Q-T from Chisocheton ceramicus. J Nat Med 77:596–603
Nugroho AE, Okabe M, Hirasawa Y, Wong CP, Kaneda T, Tougan T, Horii T, Morita H (2021) A novel trimeric triterpene from Chisocheton ceramicus Miq. Nat Prod Commun 16:1934578X211053202
Nugroho AE, Hashimoto A, Wong C-P, Yokoe H, Tsubuki M, Kaneda T, Hadi AHA, Morita H (2018) Ceramicines M-P from Chisocheton ceramicus: isolation and structure–activity relationship study. J Nat Med 72:64–72
Iijima C, Wong CP, Nugroho AE, Sotozono Y, Someya S, Hirasawa Y, Kaneda T, Hadi AHA, Morita H (2016) Anti-melanin deposition activity of ceramicines from Chisocheton ceramicus. J Nat Med 70:702–707
Wong CP, Kaneda T, Hadi AHA, Morita H (2014) Ceramicine B, a limonoid with anti-lipid droplets accumulation activity from Chisocheton ceramicus. J Nat Med 68:22–30
Wong CP, Deguchi J, Nugroho AE, Kaneda T, Hadi AHA, Morita H (2013) Ceramicines from Chisocheton ceramicus as lipid-droplets accumulation inhibitors. Bioorg Med Chem Lett 23:1786–1788
Wong CP, Shimada M, Nugroho AE, Hirasawa Y, Kaneda T, Hadi AHA, Osamu S, Morita H (2012) Ceramicines J-L, new limonoids from Chisocheton ceramicus. J Nat Med 66:566–570
Wong CP, Shimada M, Nagakura Y, Nugroho AE, Hirasawa Y, Kaneda T, Awang K, Hadi AHA, Mohamad K, Shiro M, Morita H (2011) Ceramicines E–I, new limonoids from Chisocheton ceramicus. Chem Pharm Bull 59:407–411
Mohamad K, Hirasawa Y, Litaudon M, Awang K, Hadi AHA, Takeya K, Ekasari W, Widyawaruyanti A, Zaini NC, Morita H (2009) Ceramicines B-D, new antiplasmodial limonoids from Chisocheton ceramicus. Bioorg Med Chem 17:727–730
Mohamad K, Hirasawa Y, Lim CS, Awang K, Hadi AHA, Takeya K, Morita H (2008) Ceramicine A and walsogyne A, novel limonoids from two species of Meliaceae. Tetrahedron Lett 49:4276–4278
Nugroho AE, Nakajima S, Wong CP, Hirasawa Y, Kaneda T, Shirota O, Tougan T, Horii T, Hadi AHA, Morita H (2022) Walsogynes H-O from Walsura chrysogyne. J Nat Med 76:94–101
Nugroho AE, Hirasawa Y, Kaneda T, Shirota O, Matsuno M, Mizukami H, Morita H (2021) Triterpenoids from Walsura trichostemon. J Nat Med 75:415–422
Nugroho AE, Okuda M, Yamamoto Y, Wong CP, Hirasawa Y, Kaneda T, Shirota O, Hadi AHA, Morita H (2017) Apowalsogynes A and B, two highly oxidized 3,4-Seco-apotirucallane triterpenoids from Walsura chrysogyne. Nat Prod Commun 12:1189–1192
Nugroho AE, Okuda M, Yamamoto Y, Hirasawa Y, Wong C-P, Kaneda T, Shirota O, Hadi AHA, Morita H (2013) Walsogynes B – G, limonoids from Walsura chrysogyne. Tetrahedron 69:4139–4145
Nugroho AE, Tange M, Kusakabe S, Hirasawa Y, Shirota O, Matsuno M, Mizukami H, Tougan T, Horii T, Morita H (2022) Chukranoids A-I, isopimarane diterpenoids from Chukrasia velutina. J Nat Med 76:756–764
Trager W, Jensen J (1976) Human malaria parasites in continuous culture. Science 193:673–675
Lambros C, Vanderberg JP (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420
Toya Y, Tougan T, Horii T, Uchihashi K (2021) Lysercell M enhances the detection of stage-specific plasmodium-infected red blood cells in the automated hematology analyzer XN-31 prototype. Parasitol Int 80:102206
Tougan T, Toya Y, Uchihashi K, Horii T (2019) Application of the automated haematology analyzer XN-30 for discovery and development of anti-malarial drugs. Malar J 18:8
Tougan T, Suzuki Y, Itagaki S, Izuka M, Toya Y, Uchihashi K, Horii T (2018) An automated haematology analyzer XN-30 distinguishes developmental stages of falciparum malaria parasite cultured in vitro. Malar J 17:59–59
Acknowledgements
The authors would like to thank Prof. Masatsugu Kimura (Osaka City University, Osaka, Japan) for the kind gift of the 3D7 strain, Mr. Yuji Toya and Dr. Kinya Uchihashi (Sysmex) for the setting of the XN-30 analyzer, and Ms. Toshie Ishisaka and Ms. Sawako Itagaki for their technical assistance. This work was partly supported by JSPS KAKENHI (JP 19K07152 and JP 22K06671 to MH), (JP 16K08309 to AEN), and (JP 22K06685 to YH), Japan.
Funding
This work was partly supported by JSPS KAKENHI (JP 19K07152 and JP 22K06671 to MH), (JP 16K08309 to AEN), and (JP 22K06685 to YH), Japan.
Author information
Authors and Affiliations
Contributions
AEN, TK, TK, Y, TT, TH, AHAH, and HM conceived and designed the experiments; AEN, CPW, YH, TK, and HM wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing final interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nugroho, A.E., Komuro, T., Kawaguchi, T. et al. Ceramicines U–Z from Chisocheton ceramicus and structure–antimalarial activity relationship study. J Nat Med 78, 68–77 (2024). https://doi.org/10.1007/s11418-023-01746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01746-2