Abstract
Numerous studies have investigated the fundamental mechanisms by which CO2 flooding can increase oil production by altering the properties of the hydrocarbon fluid, including oil swelling, viscosity and interfacial tension reductions, and the extraction of light-to-intermediate components. However, the interactions between CO2 and hydrocarbon fluid may also cause several problems, such as asphaltene precipitation due to crude oil's instability during the CO2 flooding process. This study investigates the complex factors that affect the instability of crude oil, including CO2 injection pressures, temperatures, and crude oil compositions. The light-dead oil samples taken from two Indonesian oil fields were used. The impacts of the instability of crude oil on CO2 displacement performance were also observed to evaluate oil recovery and minimum miscibility pressure (MMP). The observation was performed using a slim tube under varying CO2 high-pressure injections at 90 °C and 70 °C. The produced oils were analyzed based on their polarity component, saturates, aromatics, resins, and asphaltenes fractions, to observe the changes in oil composition and colloidal index instability. The results showed that increasing temperatures at given pressures resulted in higher oil recovery. Moreover, the asphaltene and resin fractions in the oil produced at a lower temperature significantly decrease compared to those at a higher temperature. It was also shown that asphaltene tends to precipitate more easily at a lower temperature. The other phenomenon revealed that the lighter oil resulted in a lower recovery than the heavier oil at a given pressure and temperature and correspondingly higher MMP. It was also suggested that CO2 flooding is more likely to cause asphaltene precipitation in light oils.
Similar content being viewed by others
Introduction
Oil production has declined over the years, and energy companies are searching for innovative methods to enhance recovery (Hosseini et al. 2021). One such method is CO2 flooding, which has been successfully proven to increase oil production (Cao and Gu 2013a; Hartono et al. 2021; Rosiani et al. 2022; Zhang et al. 2018). When CO2 is injected into a reservoir, it physically and chemically interacts with the reservoir rock and the existing hydrocarbon fluid. These interactions are the fundamental mechanisms that explain why and how CO2 injection recovers the remaining oil place. The fundamental mechanisms that occur when the CO2 interacts with the hydrocarbon fluid are oil volume swelling, oil viscosity and density reduction, as well as a decrease in CO2 – Oil interfacial tension (IFT), and vaporization-extraction phenomenon (Dindoruk et al 2020; Cao and Gu 2013b; Siagian and Grigg 1998; Zick 1986; Holm 1986). The high solubility of carbon dioxide in oil causes it to swell, thus decreasing its viscosity and density. Oil swelling causes the coalescence of disconnected oil blubs to become connected, making them a continuous phase that can be mobilized (Rezk and Foroozesh 2019).
Many studies have investigated CO2 flow mechanisms and its interactions with hydrocarbon fluid in porous media, including the oil volume swelling, vaporization/extraction phenomena, and interfacial tension (IFT) reduction. However, only a few investigated the effects of CO2–hydrocarbon fluid interactions on crude oil stability and its impact on oil recovery. Moreover, the effects of mutual interactions between crude oil and CO2 on EOR mechanisms are not well understood (Zanganeh et al. 2012). The major components of crude oil are divided into saturate, aromatic, resin, and asphaltene (SARA) components (Ashoori et al. 2017; Fakher et al. 2020; Mohammed et al. 2021). These components are all stabilized and homogenized in solution to form crude oil under normal conditions (Alimohammadi et al. 2019; Fakher and Imqam 2019). The crude oil stability depends on the characteristic interaction between its components, asphaltenes and resin (Xiong et al. 2020). Crude oil stability has been proven to be experimentally affected by several parameters, including pressure, temperature, mixture characteristics, and the number of precipitants. The injection of a solvent, such as CO2, into the hydrocarbon fluid also leads to disturbance and changes the crude oil thermodynamics (Fakher et al. 2020; Guzmán et al. 2017; Mohammed et al. 2021). Consequently, it causes asphaltene precipitation and deposition due to the disturbance of crude oil thermodynamics by CO2 injection.
Rezk and Foroozesh (2019) investigated the effect of fluid interactions of CO2 and light oil systems on swelling/extraction and interfacial tension (IFT) reduction mechanisms. They also observed that extracting the light and intermediate components by CO2 causes crude oil instability, leading to higher possibilities of precipitation asphaltene in a porous media. However, they did not investigate the effect of CO2 flooding on crude oil stability and its impact on oil recovery. Maqbool et al. (2011) investigated the effect of temperature on the asphaltene destabilization from crude oils. They concluded that the solubility of asphaltene in crude is higher at higher temperatures. This is due to the fact that a change in temperature tends to alter viscosity. The experiments conducted by these studies only focused on the effect of temperature on crude oil stability without the addition or injection of solvents such as CO2. They also did not perform the oil recovery. Cao and Gu (2013a, b) studied the CO2 flow mechanism using core flood and asphaltene precipitation due to CO2 flooding. Their work was focused on oil recovery rather than CO2 flow mechanisms and hydrocarbon fluid interactions that led to asphaltene precipitation. Kazemzadeh et al. (2015) studied asphaltene precipitation during gas injection to oil recovery by interfacial tension. They concluded that asphaltene precipitation, which occurs during gas injection, would increase the MMP of an oil–gas system. The asphaltene precipitation that occurred during gas injection indicates that the CII value is higher than 0.9, which is considered unstable (Mohammed et al. 2021). Lashkarbolooki et al. (2017) investigated the minimum miscibility pressure of CO2 and crude oil during CO2 injection in the reservoir. Their experimental results showed that for crude oil-containing asphaltene and resin, the interfacial tension can be lower for heavy crude oil with high asphaltene content. It would affect the miscibility of CO2 and crude oil because interfacial tension is one of the fundamental mechanisms of CO2 and crude oil miscibility. Ashoori et al. (2017) studied the relationship between crude oil compositions and its stability based on SARA fractions. The crude oil stability was investigated by using SARA analysis to observe the different components of seven crude oil types. Their investigations found that the stability of crude oil depends on all these components and cannot be associated only with one of them individually. Each fraction of SARA influences the stability of crude oil. Their study was focused only on the compositions of crude oil based on SARA components and preliminary screening to detect problems associated with asphaltene. Fakher and Imqam (2019) investigated the flow mechanism of CO2 flooding in nanopores and the factors that affect oil recovery. They observed the effects of CO2 injection pressure, temperature, oil viscosity, soaking time, and porous media thickness on the oil recovery and the possibility of asphaltene precipitation. They showed that those parameters have an impact on oil recovery and tend to form asphaltene precipitation. Nevertheless, they did not observe the change in crude oil stability due to CO2 flooding based on SARA components, alongside its impact on oil recovery performance. Asphaltene precipitation can be predicted by the colloidal and solubility approach, such as the colloidal instability index (CII), which views asphaltene as a colloidal peptized by resins in the oil mixture (Mohammed et al. 2021; Mullins 2008). Moreover, asphaltene precipitation impacts oil recovery and thus would affect minimum miscibility pressure (MMP) measurement.
Much research has been conducted to investigate the mechanisms of CO2 and hydrocarbon fluid interactions on oil swelling, extraction of light and intermediate oil components, reduction in oil viscosity, and CO2–oil interfacial tension reduction. These phenomena are directly caused by CO2 dissolution into crude oil. However, little research has been conducted to investigate the mechanisms of CO2 and hydrocarbon fluid interactions on crude oil stability and its impact on oil recovery. Sarma (2003) and Alimohammadi et al. (2019) also reported that asphaltene problems due to CO2 injection occurred more easily from light oil than from heavy oil. It is important to note that asphaltene problems tend to occur if the equilibrium condition of crude oil is disturbed. Furthermore, light oil reservoirs are more often become candidates for gas or CO2 injection. This study aims to investigate the effects of CO2 and crude oil interactions on crude oil stability and its impact on oil recovery performance. Understanding the effects of CO2 flooding on crude oil stability and recovery performance on the enhanced oil recovery (EOR) mechanism is crucial because it can help the petroleum industry optimize their CO2 flooding implementation and forecast any possible problems due to the interactions between CO2 and crude oil, such as asphaltene problems. The factors or parameters studied include CO2 injection pressure, temperature, and crude specific gravity. This study was conducted in different temperatures and crude samples. Then the oil recovery was measured from the slim tube apparatus. The crude oil stability was observed based on the results of the SARA analysis.
CO2 flooding and crude oil stability: overview
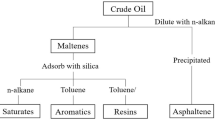
Crude oils can be described compositionally by several methods. One simple compositional analysis scheme is to divide an oil into its saturate, aromatic, resin, and asphaltene fractions, commonly referred to as SARA (Fan et al. 2002). SARA stands for saturates, aromatics, resins, and asphaltenes. SARA analysis divides crude oil components based on their polarity. Saturates are the compounds in the hydrocarbon that are saturated and nonpolar. Saturates also do not contain double bonds (Fakher et al. 2020). Saturated hydrocarbons are referred to as alkanes, including normal alkanes, branched alkanes, and cyclo alkanes. Methane is the most basic alkane compound, followed by ethane and propane (Goel et al. 2017). Aromatics compounds are slightly more complex in structure than saturates. In general, they are nonpolar and possess an unsaturated hydrocarbon ring with multiple carbon–carbon double bonds within the ring structure, for example, toluene, xylene, and phenolic acid (Liao et al. 2019; Punase et al. 2016). Since aromatics are typically nonpolar, they are not very stable with asphaltene, which is typically a highly polar compound. Resins are peptizing agents and play significant roles in stabilizing asphaltene in crude oil (Leontaritis and Mansoori 1988). Resins have both a polar side and a nonpolar side. Thus, the role of resins as a bridging material that connects the nonpolar hydrocarbon to the highly polar asphaltene (Miadonye and Evans 2010). They have a higher molecular weight than saturates and aromatics.
Asphaltenes are defined as the fraction of crude oil that is insoluble in normal alkanes. They are considered one of the most complex components of crude oils. Asphaltenes are highly polar and complex molecules, in contrast to crude oil as it is. Asphaltenes are insoluble in light n-alkanes such as n-pentane or n-heptane (Fakher et al. 2020; Kokal and Sayegh 1995; Mullins 2008; Soleymanzadeh et al. 2019). The molecular structure of each SARA fractions, including asphaltenes, refers to the studies conducted by Alimohammadi et al. (2019), Fakher et al. (2020), and Subramanian et al. (2016). All these SARA components are stabilized in solution to form crude oil under normal conditions. If the equilibrium condition of crude oil is disturbed by solvent injection or pressure depletion, it leads to asphaltene precipitation or asphaltene destabilization (Fakher and Imqam 2019). The terms asphaltene destabilization refers to the growth of nanoscale asphaltene. The subsequent process is aggregation, which represents asphaltene particles on a microscale (Chaisoontornyotin et al. 2016). The final stage is the precipitation process, which entails the transition from nanoscale particles to microscale particles. Asphaltene destabilization is affected by a number of parameters, including pressure, temperature, crude oil mixture characteristics, and precipitants properties (Alimohammadi et al. 2019). The destabilization of asphaltenes in crude oils can have a negative impact on the performance of production systems and lead to reduced recovery efficiencies, both upstream and downstream. For instance, blockage of pore throats, alteration of reservoir wettability, and a decrease in reservoir permeability are deleterious effects of asphaltene precipitation and deposition. In addition, asphaltene precipitation/deposition in the downstream portion results in the clogging of flow facilities, the formation of solids in storage containers, and the fouling of safety valves (Kokal and Sayegh 1995; Sarma 2003; Zanganeh et al. 2012). Therefore, understanding the effects of CO2 flooding on crude oil stability is crucial to forecast of any possible problems due to CO2 flooding implementation.
The two approaches widely used to describe asphaltene behavior are the colloidal and solubility approaches. The colloidal approach considers asphaltene to be a colloid peptized by the presence of resins in the oil mixture and implies that the precipitation is irreversible (Mullins 2008). The concept of asphaltene's colloidal existence has been intensively studied and widely used (Guzmán et al. 2017). Some of the tests currently used to determine the stability of crude oils, which applied the colloidal approaches, based on SARA fraction are colloidal instability index (CII), colloidal stability index (CSI), and Stankiewics plot (SP). This study used colloidal instability index (CII) for screening criterion to identify the possible asphaltene precipitation phenomenon. As mentioned, asphaltene stability is a function of saturates, aromatics, and resins (Guzmán et al. 2017). Then, colloidal instability index (CII) is mathematically expressed as a ratio of the sum of saturates and asphaltenes (flocculants) to the sum of resins and aromatics (peptizers), as stated in Eq. 1. The lower the value of CII, the higher asphaltene stability in crude oils. According to Asomaning and Watkinson (2000), if CII values are less than 0.7 (\(\mathrm{CII}\le 0.7)\), it is considered stable. If the CII values are greater than 0.9 (\(\mathrm{CII}\ge 0.9)\), the asphaltene fraction tends to be unstable within the crude oil. Stankiewics plot (SP) is a cross-plot to determine asphaltene precipitation tendency, and it is also perceived as a quick evaluation tool (Mohammed et al. 2021; Saboor et al. 2022). This method considers the SARA fraction, and the cross-plot is divided into two regions. The regions consist of the stable and unstable regions, when asphaltene/resins ratios (A/R) are less than 0.5 irrespective of saturates/aromatics (S/Ar) ratio, the region is considered to be stable as reported by Bahrami et al. (2015) and Sulaimon et al. (2020). Stankiewics plots are often combined with the CII to obtain reliable results.
Materials and methods
This study's experiment design and method are intended to accomplish the research objectives. This study aims to investigate the mechanisms of CO2 and crude oil interactions on crude oil stability and its impact on recovery performance. Therefore, to achieve the research objective, several parameters were studied to investigate the effects of those parameters on crude oil stability and their impact on CO2 flow mechanism in porous media. This study was conducted at different pressures, temperatures, and crude samples. Then the CO2 flow mechanism in porous media and oil recovery were observed from the slim tube apparatus. The stability of crude oil was observed based on the results of the SARA analysis.
Experimental apparatus
The experiment was conducted using CO2 displacement slim tube apparatus STS 700 by Vinci Technologies, which has a silica sand pack design with a pore volume of 120 mL. The slim tube tests have been widely accepted and used by the petroleum industry as a standard method for CO2 displacement in porous media and for the minimum miscibility pressure (MMP) measurement (Abdurrahman et al. 2015; Zhang et al. 2019; Zhang and Gu 2015). The slim tube apparatus is considered to be the most accurate way to determine the minimum miscibility pressure (Adel et al. 2016). Therefore, in this study, the slim tube apparatus was considered to determine the minimum miscibility pressure.
This slim tube systems comprise of the following hardware features: a floating piston high-pressure accumulator for oil, a floating piston high-pressure accumulator for gas, a floating piston high-pressure accumulator for solvent, a high-pressure positive displacement syringe pump, a Hastelloy coil tube (slim tube), air bath for temperature control, back-pressure regulator, liquid measurement system, wet gas meter, a workstation for remote control, and high-pressure visual cell. The slim tube experiment was validated by simultaneous density measurement which integrated to the coil tube and computer system. Therefore, an additional densitometer was fitted to monitor the effluent fluid density from the slim tube. The effluent liquid volumes, density, and pressure drop are continuously monitored using a digital volume measuring detector or sensor and recorded in an integrated computer system. This slim tube has the ability to test the dynamic miscibility at reservoir conditions with a maximum pressure of 10,000 Psi and a maximum temperature of 150 °C. The specification of this slim tube system and schematic diagram of the slim tube is shown in Table 1 and Fig. 1, respectively.
Materials
The crude oil samples were collected from the JTB and RDG Indonesian oilfields with a specific gravity of 36.4oAPI and 42.6oAPI, respectively. The crude oil samples, JTB and RDG, were light dead oil samples taken from stock tanks with densities of 810.26 kg/m3 and 802.2 kg/m3, respectively, measured under ambient conditions at a standard temperature of 15.5 °C (60°F). In addition, the average reservoir temperature of the field was 90 °C. The crude oil samples' compositions were analyzed using gas chromatography Agilent 7890B GC System, and the results are shown in Table 2. The compositions of the crude oil samples were also analyzed based on their polarity, consisting of saturates, resins, aromatics, and asphaltenes (SARA) components, as shown in Table 3. The procedures for quantifying the SARA fractions followed the American Society for Testing and Materials (ASTM) method, which will be explained in the following section. The CO2 used for the slim tube experiments was ultra-high pure CO2 (99.9%).
Experimental procedures
The main experiment in this study was CO2 flooding using a dynamic slim tube test. Prior to conducting the slim tube tests, the hydrocarbon composition of the crude oil samples was analyzed using gas chromatography. The composition of the crude oil samples was further analyzed based on their polarity components which contain SARA contents. The asphaltene content of crude oil was measured by following the American Society for Testing and Materials method ASTM D6560, also known as IP-143 (El Nagy et al. 2022; Santos et al. 2019). In summary, for this method, a mass portion of crude oil was mixed with a volume of the precipitant agent (n-heptane) under hot reflux. The mixture was then filtered using the specified filter paper. Then, the solid fraction was washed with heated heptane under reflux to remove residual maltenes from the asphaltene particles. The asphaltene was separated from any precipitated inorganic material by hot toluene dissolution. The ASTM D6560 method has some limitations, such as being time-consuming and using relatively large amounts of solvent. In addition, the ASTM D6560 method covers the quantification range of 0.5–30% asphaltene (Kharrat et al. 2013; Rogel et al. 2009). Therefore, the repeatability and reproducibility of this method are 10% and 20%, respectively. The consideration of applying the ASTM D6560 for quantifying the asphaltene content in this study is due to the use of light crude oil samples, which have a small quantity of asphaltene content. SARA analysis is important for the preliminary screening of crude oil instability. The results of SARA analysis conducted on original crude oil samples in weight percentages are shown in Table 3. It is important to identify and conduct preliminary screening on crude oil instability by calculating the colloidal instability index (CII) (Ashoori et al. 2017). CII, which is based on the input of SARA fractions, is defined as the ratio of the sum of asphaltene and saturates fractions to resins and aromatics fraction (Ashoori et al. 2017; Guzmán et al. 2017; Mohammed et al. 2021), as stated in Eq. 1. As mentioned, Crude oil with CII values less than 0.7 (\(\mathrm{CII}\le 0.7)\) is considered as stable. If the CII value is greater than 0.9 (\(\mathrm{CII}\ge 0.9)\) is considered unstable. After measuring and analyzing the composition of the original crude oil samples, the next step was to prepare for the slim tube experiment.
Slim tube experiment
As mentioned, a slim tube experiment is well known and used by the petroleum industry as a standard method for CO2 displacement in porous media and for the minimum miscibility pressure (MMP) measurement (Abdurrahman et al. 2015). The slim tube experiment was conducted in three stages: preparation, cleaning, oil saturation stages, and CO2 injection. The slim tube system needs to be cleaned first before it is saturated with oil. The preparation stage includes cleaning the existing fluid, washing and drying liquid-saturated parts, and reassembling the system. These activities were followed by filling the three accumulators with an oil sample, ultra-high pure CO2, and solvent, with the system set at the desired pressure and a stable reservoir temperature of 90 °C.
Two pore volumes (2 PV) of toluene were injected into the coil tube, followed by two pore volumes (2 PV) of supercritical CO2 to ensure that the porous medium inside was clean and dry. The coil tube was then weighed and compared to its initial weight to ensure that it was fully clean and dry. If the weight discrepancy exceeds one gramme, the cleaning and drying process must be repeated. After the coil tube was cleaned and verified, it was saturated by injecting an oil sample. At the same time, the back pressure gradually increased until the desired test pressure was reached. It is noteworthy that a back pressure regulator was included to maintain constant pressure inside the system. In the first run, the pressure was set and maintained at 1000 psi. After stabilizing the pressure and temperature of the system, two pore volumes of the crude sample were injected to ensure the coil tube was fully saturated. The details of the slim tube experiment procedures refer to Adel et al. (2016) and Abdurrahman et al. (2015).
The next stage was the injection of CO2 into the slim tube, and the effluent fluids were observed visually from the visual cell separator. The effluent fluids were also measured along the duration of the experiment, which were recorded and stored in an integrated computer data acquisition system to calculate the recovery factor at each pressure. An additional densitometer was also fitted to monitor the effluent fluid density from the slim tube. The data obtained from the experiment, such as the inlet and outlet pressure, differential pressure, effluent oil volume, effluent density, and temperature, were stored in an integrated computer data acquisition system. The oil recovery factor for each pressure was calculated, and the results were plotted to determine the MMP. Six to seven runs were conducted with different displacement pressures to ensure consistency. In all cases, the displacement pressures were performed at 1000, 2000, 2500, 3000, 3500, 4000, and 5000 Psi.
Furthermore, MMP obtained from the distinct point of maximum curvature at an oil recovery of 1.2 PV gas injected was plotted against pressure (Glaso 1990; Hudgins et al. 1990) or near maximum recovery in a series of displacements (Yellig and Metcalfe 1980). The final stage was cleaning oil samples in the slim tube by injecting toluene as a solvent. It is also important to note that these procedures were applied at a reservoir temperature of 90 °C. The composition of oil samples in the slim tube's outlet separator was also analyzed using a gas chromatograph to observe the extraction of hydrocarbon components at each pressure. Furthermore, the compositions of asphaltene fractions were observed from the residual oil/effluent fluid at each CO2 injection pressure using ASTM D6560 method as stated above. The slim tube experiment was re-conducted at a temperature of 70 °C to investigate and observe the effects of temperature on crude oil stability and its impact on oil recovery performance.
Results and discussions
As mentioned earlier, understanding the effects of CO2 flooding on crude oil stability and recovery performance on the enhanced oil recovery (EOR) mechanism is crucial because it can help the petroleum industry optimize their CO2 flooding implementation and forecast any possible problems due to the interactions between CO2 and crude oil, such as asphaltene problems. The effects of the interactions between CO2 and crude oil on crude stability and recovery performance will be explained and discussed in this section. The CO2 flow mechanism in porous media is presented, including the impact of crude oil instability due to CO2 flooding on recovery performance and minimum miscibility pressure (MMP) measurement. Subsequently, factors that can disrupt the stability of crude oil, including CO2 injection pressure, temperature, and different crude oil samples, are discussed in this section.
CO2 flooding flow mechanism in porous media
The CO2 flooding experiment was conducted using slim tube apparatus with seven runs in different pressures and different temperatures. The displacement process was performed at high operating pressures of 1000, 2000, 2500, 3000, 3500, 4000, and 5000 Psi. All displacement processes were conducted at the temperature of 90 °C, which is the average reservoir temperature, and re-conducted at the temperature of 70 °C which is the average temperature of the surface and the reservoir in the field.
Impacts crude oil instability due to CO2 flooding on oil recovery performance
The oil recovery performance from the CO2 displacement process was measured using the slim tube experiment. The determination of minimum miscibility pressure (MMP) was also estimated by oil recovery against injection pressure, as referred to (Glaso 1990; Hudgins et al. 1990; Yellig and Metcalfe 1980). Table 4 shows the oil recovery results against CO2 injection pressures on crude oil samples at operating temperatures of 70 °C and 90 °C. Table 4 further shows that the oil recovery obtained for both crude oil samples at operating temperature 70 °C is lower than at 90 °C. The maximum oil recovered at a temperature of 70 °C for RDG and JTB crude oil samples are 85.7% and 89%, respectively. Meanwhile, at a temperature of 90 °C for RDG and JTB crude samples are 93.5% and 94.5%. Increasing temperature resulted in increased oil recovery. As earlier mentioned, an increase in temperature causes a decrease in viscosity, and it is the reason behind the increase in oil recovery at a higher temperature. Colloidal Index Instability (CII) result showed that it is higher at a temperature of 70 °C than 90 °C. Furthermore, asphaltene wt.% precipitates more at a lower temperature, as shown in Tables 6, 7, 8 and 9.
The other phenomenon in these experiment results was that the RDG crude sample (42.6oAPI), lighter than the JTB crude sample (36.4oAPI), resulted in lower oil recovery than the JTB crude sample for either operating temperature at 70 °C or 90 °C. After the CO2 injection, CII results of RDG crude sample are also much higher than the JTB. It indicates that asphaltene tends to be easily precipitated when the CO2 is injected into the RDG crude sample than JTB, even though RDG is lighter than JTB. Therefore, oil recovery from the CO2 displacement process involving RDG crude is lower than JTB. It should be noted that the asphaltene content in JTB crude is higher than in RDG, as shown in Table 3.
Impacts crude oil instability on minimum miscibility pressure (MMP) measurement
Minimum miscibility pressure (MMP) is obtained from a distinct point of maximum curvature when oil recovery at 1.2 PV gas injected is plotted against pressure or very near maximum recovery in a series of displacements using the slim tube apparatus. As mentioned, the determination of the MMP from slim tube tests refers to the studies conducted by Glaso (1990), Hudgins et al. (1990), and Yellig and Metcalfe (1980). MMP results from the slim tube tests for RDG and JTB crude oil samples are shown in Figs. 2 and 3, respectively. The slim tube test results in Fig. 2 show that the MMP value for RDG crude oil sample for the temperature of 90 °C and 70 °C is 2639 Psi and 3517 Psi, respectively. In contrast to empirical correlations, as Desouky et al. (2017) stated, a higher temperature causes a higher MMP value and vice versa. Lashkarbolooki and Ayatollahi (2018) and Yellig and Metcalfe (1980) also reported that the MMP increased with the temperatures. Lashkarbolooki and Ayatollahi (2018) reported that MMP for crude oil with an APIo of 35 and low asphaltene content (about 0.1 wt.%) and diesel fuel show increased with the temperatures for both systems. The linear increase of MMP with temperature for heptane, hexadecane, light crude oil with APIo of 38 and 35 was also reported by Lashkarbolooki et al. (2017). On the other hand, the MMP result for RDG crude sample at 70 °C is higher than at 90 °C. This phenomenon requires a comparison of the MMP values of various crude oils and temperatures to analyze the effect of temperature and oil composition on the MMP values. Table 5 compares measured MMP values from various crude oil types and temperatures. Interestingly, some of the data show that crude oil with lower temperatures has a higher MMP value, similar to RDG crude. The same observation was also reported by Hagedorn and Orr (1994). According to Hagedorn and Orr (1994), significant quantities of multi-ring aromatic components in crude oil are disadvantageous to developing miscibility with CO2. Multi-ring aromatic components are extracted less efficiently by dense CO2. Therefore, it decreases slim tube recovery at a given pressure and correspondingly higher MMP. Based on the composition of RDG crude oil, as shown in Table 3, the aromatic component of RDG is higher than the JTB crude. Also, in contrast, resin fraction has a significant role in the stability of asphaltene in crude oil (Lashkarbolooki et al. 2017; Mohammed et al. 2021).
This study demonstrates that experimental results suggest that crude oil instability, which is shown by asphaltene precipitation, significantly affects oil recovery performance and the MMP measurement. The same observation was also shown by Kazemzadeh et al. (2015). The authors reported that asphaltene precipitation, which could occur during gas injection, would increase the MMP of an oil–gas system. Although CO2 dissolution reduces the interfacial tension (IFT) values at the high-pressure range, accumulation of asphaltene at the interface of the oil phase and CO2 occurs, increasing the interfacial tension. In other words, asphaltene precipitation due to gas injection increases the MMP of an oil and gas system. Moreover, temperature changes also contribute to the formation of asphaltene precipitation in porous media (Maqbool et al. 2011). This phenomenon is caused by the change in viscosity with temperature. Therefore, temperature changes will affect the stability of crude oil and impact recovery performance and MMP. The effects of temperatures on crude oil stability will be discussed in more detail in "Effects of temperature on crude oil stability" section.
The MMP results of JTB crude oil at a temperature of 90 °C and 70 °C are 3299 Psi and 3066 Psi, respectively, as shown in Fig. 3 and Table 4. Based on the CII results in Tables 7 and 9, JTB crude oil is more stable than RDG despite being heavier. In addition, the asphaltene content in JTB crude oil is higher than in RDG sample. MMP results of JTB crude oil aligned with the empirical correlations that the higher the temperature, the higher the MMP.
The overall results indicated that changes in pressures, temperatures, and oil composition due to CO2 flooding have a significant impact on crude oil stability. Therefore, this affects the oil recovery performance and MMP measurement by the CO2 displacement process. Crude oil stability also strongly depends on all the crude oil composition (saturates, aromatics, resins, and asphaltenes) behavior and the percentage of each component, as shown in Table 3. It cannot be associated only with one of them individually. Furthermore, temperature has a significant impact on asphaltene precipitation, crude oil instability, and oil recovery performance.
Effects of CO2 flooding on crude oil stability
The stability of crude oil was analyzed based on SARA fractions and CII, calculated as shown in Eq. 1. As earlier mentioned, crude oil stability is affected by several parameters, such as pressure, temperature, mixture characteristics, and the number of precipitants.
Effects of CO2 injection pressure on crude oil stability
Four high-pressured CO2 injection pressures were investigated in this research, including 1000, 2000, 3000, and 4000 psi for two crude oil samples RDG (42.6oAPI) and JTB (36.4oAPI). The weight percentages (wt.%) of resin fractions, asphaltene fractions, and CII at temperature 70 °C for crude sample RDG and JTB are shown in Tables 6 and 7, respectively. Meanwhile, Fig. 4 shows the compositional analysis of the initial RDG crude oil sample and the remaining oil with increasing CO2 injection pressures. Figure 4 shows that the light-intermediate component of the RDG crude oil decreases with increasing injection pressures. It indicates that the saturate components in RDG crude oil also decrease with increasing CO2 injection pressures. The saturate components in crude oil are not polar materials, they do not contain any double bond, and are generally referred to as alkanes. The decreasing saturate components with the increasing injection pressure could be due to the extraction of light and intermediate hydrocarbon by dense CO2. It is important to note that the density of CO2 increases with increasing pressure. Thus, the extraction of hydrocarbons from oil also increases as the density of CO2 increases (Dindoruk et al. 2020). According to Hagedorn and Orr (1994), branched alkanes and normal alkanes of a given carbon number are easily extracted.
The relationship between resin, asphaltene fractions, and CII results in pressures for crude sample RDG and JTB at temperature 70 °C can also be seen in Figs. 5 and 7, respectively. Figures 5 and 7 show that resin and asphaltene fractions decrease with increasing CO2 injection pressure. In contrast to the CII results, it shows a significantly increasing index with decreasing resin and asphaltene contents. As earlier mentioned, if the CII is higher than 0.9, it is considered unstable. Resin plays a significant role in the stability of crude oil because it is characterized by polar and nonpolar attributes used for bridging and peptizing agents connected to the nonpolar hydrocarbons compound and the highly polar asphaltene (Leontaritis and Mansoori 1988; Miadonye and Evans 2010). These results indicated that the decrease in weight percent of the resin component causes crude oil instability, indicated by increasing the CII.
The asphaltene contents in Table 6 and Fig. 5 also decrease with increasing pressure. It indicates that asphaltene was precipitated in the porous media. Asphaltene precipitation occurs due to the reduction of the resin content, which has the role of peptizing agents for the asphaltenes stability in crude oil. Prakoso et al. (2017) stated that the ratio of asphaltene and resin is critical because of its impact on asphaltene stability. Deo and Parra (2012) also stated that multiple contacts of CO2 and crude oil cause asphaltene precipitation in the formation.
Table 7 shows weight percentages of resin and asphaltene fractions results for JTB (36.4oAPI) crude oil sample at a temperature of 70 °C under various pressures. Similar to RDG crude sample, the saturate fraction in the JTB crude sample decreases due to the extraction of light-intermediate components, as shown in compositional analysis in Fig. 6. Meanwhile, resin and asphaltene fractions decrease with increasing pressure. The decreasing resin and asphaltene content could be due to some wt.% of asphaltene precipitates into porous media.
The CII index shows a slight increase with the increasing CO2 injection pressure. It is shown in Table 7 and Fig. 7 that CII index tends to be constant within the range of index 2.0 to 2.3. In other words, CII of the JTB crude sample at a temperature of 70 °C is more stable than RDG crude sample. This is because the resin fraction as the peptizing agents in JTB crude sample is higher than in the RDG crude sample. Therefore, the stability of the JTB crude sample is more stable than the RDG.
Effects of temperature on crude oil stability
The CO2 flooding experiments were re-conducted at an operating temperature of 90 °C to observe the effect of temperature on crude oil stability and the impact on oil recovery. Temperature is an important parameter for the stability of asphaltenes in crude oil (Maqbool et al. 2011). The wt.% of resin, asphaltene fraction, and CII results obtained at temperature 90 °C for RDG and JTB crude samples are shown in Tables 8 and 9, respectively. In addition, the relationship between resin, asphaltene fractions, and CII at temperature 90 °C for RDG and JTB crude samples are also shown in Figs. 8 and 9, respectively.
Table 8 and Fig. 8 show a similar trend result at the temperature of 70 °C. Compared to CII results obtained at a temperature of 70 °C in Table 6 and Fig. 5, CII at the temperature of 90 °C is lower than at 70 °C. It indicates that at a temperature of 90 °C, crude sample is more stable than at 70 °C. At a temperature of 90 °C, resin and asphaltene decrease slightly with increasing pressures. The reduction of resin and asphaltene follows a similar trend as that resulted in a temperature of 70 °C. However, asphaltene precipitate more at a temperature of 70 °C than at 90 °C.
Table 9 shows the weight percentages (wt.%) of resin, asphaltene fractions, and CII for JTB crude sample at an operating temperature of 90 °C. Meanwhile, Fig. 9 shows the relationship between resin fractions, asphaltene fractions, CII, and CO2 injection pressure. The saturate components decrease with increasing pressure due to the extraction by dense CO2 as shown in Fig. 6 above. Otherwise, the asphaltene content decreases with increasing pressure. This indicates that there are some percentages of asphaltene precipitated in porous media. The CII results obtained after the CO2 injection into JTB crude sample at a temperature 90 °C are slightly higher than the CII results at 70 °C, as shown in Table 7 and Fig. 7.
Based on weight percentages of asphaltene fraction after CO2 injection at temperatures 70 °C and 90 °C for either RDG or JTB crude samples, it can be seen that at a lower temperature, more asphaltene precipitation occurs in porous media than at higher temperature. In other words, when the CO2 interacts with crude oil, the asphaltene stability in crude oil increases with increasing temperature. A similar observation was also reported by Hu and Guo (2001), which investigated the effect of temperature and molecular weight of n-alkane precipitants on asphaltene precipitation. This phenomenon occurs due to the relationship between temperature and viscosity. As the viscosity of the mixture decreases with increasing temperature, the effective diffusivity of the particles increases (Maqbool et al. 2011). Therefore, at higher temperatures, the lower viscosity causes the asphaltene to become more stable in crude oil. Wang et al. (2016) investigated the characteristics of produced and residual oils in CO2 flooding using a core flood. Six CO2 floods were conducted under different injection pressures and two temperatures. They reported that the asphaltene content in the produced oil collected after the CO2 breakthrough was relatively lower than the original crude oil samples. It occurs because the asphaltenes component in the crude oil tends to precipitate and deposit in the sand pack (porous media).
Conclusions
This study investigates the factors that affect the instability of crude oil during the CO2 flooding process. The impacts of the instability of crude oil on CO2 displacement performance were also observed to evaluate oil recovery and minimum miscibility pressure (MMP). According to the experimental results, the main conclusions reached from this study are shown below.
-
1.
An increased CO2 injection pressure causes saturates components, which contain light-intermediate hydrocarbon, to be extracted by dense CO2. Furthermore, the resin and asphaltene weight percent (wt.%) for the produced oil decreased with the increasing CO2 injection pressure. The decreased resin and asphaltene content in crude oil leads to instability in crude oil, which is also indicated by increasing the colloidal index instability (CII). Therefore, it leads to asphaltene precipitation in the porous media.
-
2.
Temperatures have significant effects on crude oil stability. Increasing the temperature resulted in a decrease in the colloidal instability index (CII). It indicates that crude oil tends to be more stable at higher temperatures. At a lower temperature, more asphaltenes are precipitated in the porous media than at a higher temperature. This phenomenon tends to occur due to an increase in the mixture viscosity with a decrease in temperature, leading to a decrease in the effective diffusivity of the particles.
-
3.
Increasing the temperature also leads to an increase in oil recovery. The CII results showed that crude oil is more stable at a higher temperature. The decrease in oil viscosity due to CO2 flooding with the increasing temperature is the reason behind the increase in oil recovery.
-
4.
The CO2 flooding on light oil resulted in a lower oil recovery factor than the heavier crude oil, even though the heavier oil has a much higher asphaltene content. It indicates that the crude oil instability problem and asphaltene precipitation due to CO2 flooding occur more easily in light than in heavy oil.
-
5.
The crude oil instability due to CO2 flooding significantly impacts minimum miscibility pressure (MMP) measurement. The quantities of multi-ring aromatic components in crude oil are disadvantageous to developing miscibility with CO2 because their components are inefficiently extracted by dense CO2. Therefore, it reduces the slim tube recovery at a given pressure and results in a correspondingly higher MMP. RDG crude oil has a higher multi-ring aromatic component than JTB in this case.
-
6.
Overall, this study has shown that changes in pressures, temperatures, and oil composition due to CO2 flooding significantly impact crude oil stability. These factors affect oil recovery performance and MMP measurement using the slim tube. Crude oil stability also strongly depends on the composition of the crude oil (saturates, aromatics, resins, and asphaltenes) and the percentage of each component. However, it cannot be associated with only one of them individually.
-
7.
Further studies are needed to investigate the effect of other parameters on crude oil stability during CO2 flooding, such as the number of precipitants and the rock properties of the porous media, such as permeability, porosity, and wettability, to provide more comprehensive results.
Abbreviations
- MMP:
-
Minimum miscibility pressure
- CII:
-
Colloidal index instability
- SARA:
-
Saturates, resins, aromatics, and asphaltenes
- EOR:
-
Enhanced oil recovery
- API:
-
American Petroleum Institute
- GC:
-
Gas chromatography
- PV:
-
Pore volume
- ASTM:
-
American Society for Testing and Materials
- IFT:
-
Interfacial tension
- CSI:
-
Colloidal stability index
- SP:
-
Stankiewics plot
References
Abdurrahman M, Permadi AK, Bae WS (2015) An improved method for estimating minimum miscibility pressure through condensation-extraction process under swelling tests. J Pet Sci Eng 131:165–171. https://doi.org/10.1016/j.petrol.2015.04.033
Adel IA, Tovar FD, Schechter DS (2016) Fast-slim tube: a reliable and rapid technique for the laboratory determination of MMP in CO2-light crude oil systems. In: SPE improved oil recovery conference. SPE-179673-MS. https://doi.org/10.2118/179673-MS
Alimohammadi S, Zendehboudi S, James L (2019) A comprehensive review of asphaltene deposition in petroleum reservoirs: theory, challenges, and tips. Fuel 252:753–791. https://doi.org/10.1016/j.fuel.2019.03.016
Ashoori S, Sharifi M, Masoumi M, Mohammad Salehi M (2017) The relationship between SARA fractions and crude oil stability. Egypt J Pet 26(1):209–213. https://doi.org/10.1016/j.ejpe.2016.04.002
Asomaning S, Watkinson AP (2000) Petroleum stability and heteroatom species effects in fouling of heat exchangers by asphaltenes. Heat Transf Eng 21(3):10–16. https://doi.org/10.1080/014576300270852
Bahrami P, Kharrat R, Mahdavi S, Firoozinia H (2015) Prediction of the gas injection effect on the asphaltene phase envelope. Oil Gas Sci Technol Rev. https://doi.org/10.2516/ogst/2014037
Cao M, Gu Y (2013a) Temperature effects on the phase behaviour, mutual interactions, and oil recovery of a light crude oil—CO2 system. Fluid Phase Equilib 356:78–89. https://doi.org/10.1016/j.fluid.2013.07.006
Cao M, Gu Y (2013b) Oil recovery mechanisms and asphaltene precipitation phenomenon in immiscible and miscible CO2 flooding processes. Fuel 109:157–166. https://doi.org/10.1016/j.fuel.2013.01.018
Chaisoontornyotin W, Haji-Akbari N, Fogler HS, Hoepfner MP (2016) Combined asphaltene aggregation and deposition investigation. Energy Fuels 30(3):1979–1986. https://doi.org/10.1021/acs.energyfuels.5b02427
Deo M, Parra M (2012) Characterization of carbon dioxide induced asphaltene precipitation. Energy Fuels 26(5):2672–2679. https://doi.org/10.1021/ef201402v
Desouky SM, Ramzi M, Al-Sabagh AM, Mansour EM, Zawawy FM (2017) A new estimating method of minimum miscibility pressure as a key parameter in designing CO2 gas injection process. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2017.12.002
Dindoruk B, Johns R, Orr FM (2020) Measurement of minimum miscibility pressure: a state-of-the-art review. In: SPE improved oil recovery conference. SPE-200462-MS. https://doi.org/10.2118/200462-MS
El Nagy HA, El Tamany ESH, Abbas OA, Ibrahim AZ, Mahmoud MAA (2022) Rapid and simple method for measuring petroleum asphaltenes by the centrifugation technique. ACS Omega 7(50):47078–47083. https://doi.org/10.1021/acsomega.2c06225
Fakher S, Imqam A (2019) Asphaltene precipitation and deposition during CO2 injection in nano shale pore structure and its impact on oil recovery. Fuel 237:1029–1039. https://doi.org/10.1016/j.fuel.2018.10.039
Fakher S, Ahdaya M, Elturki M, Imqam A (2020) Critical review of asphaltene properties and factors impacting its stability in crude oil. J Pet Explor Prod Technol 10(3):1183–1200. https://doi.org/10.1007/s13202-019-00811-5
Fan T, Wang J, Buckley JS (2002) Evaluating crude oil by SARA analysis. In: SPE/DOE improved oil recovery symposium. SPE-75228-MS. https://doi.org/10.2118/75228-MS
Glaso O (1990) Miscible displacement: recovery tests with nitrogen. SPE Res Eng 5(01):62–68. https://doi.org/10.2118/17378-PA
Goel P, Saurabh K, Patil-Shinde V, Tambe SS (2017) Prediction of °API values of crude oils by use of saturates/aromatics/resins/asphaltenes analysis: computational-intelligence-based models. SPE J 22(3):817–853. https://doi.org/10.2118/184391-pa
Guzmán R, Ancheyta J, Trejo F, Rodríguez S (2017) Methods for determining asphaltene stability in crude oils. Fuel 188:530–543. https://doi.org/10.1016/j.fuel.2016.10.012
Hagedorn KD, Orr FM (1994) Component partitioning in CO2/crude oil systems: effects of oil composition on CO2 displacement performance. SPE Adv Technol 02:177–184. https://doi.org/10.2118/25169-PA
Hartono KF, Permadi AK, Prakoso S (2021) The prospect of CO2 flooding and its potential application to Indonesian mature fields. In: AIP conference proceedings, vol 2363. https://doi.org/10.1063/5.0061058
Holm LW (1986) Miscibility and miscible displacement. J Pet Technol 38(08):817–818. https://doi.org/10.2118/15794-pa
Hosseini E, Sarmadivaleh M, Mohammadnazar D (2021) Numerical modelling and experimental investigation on the effect of low-salinity water flooding for enhanced oil recovery in carbonate reservoirs. J Pet Explor Prod 11(2):925–947. https://doi.org/10.1007/s13202-020-01071-4
Hu YF, Guo TM (2001) Effect of temperature and molecular weight of n-alkane precipitants on asphaltene precipitation. Fluid Phase Equilib 192:13–25. https://doi.org/10.1016/S0378-3812(01)00619-7
Hudgins DA, Uave FM, Chung FTH (1990) Nitrogen miscible displacement of light crude oil: a laboratory study. SPE Reserv Eng 5(01):100–106. https://doi.org/10.2118/17372-PA
Kazemzadeh Y, Parsaei R, Riazi M (2015) Experimental study of asphaltene precipitation prediction during gas injection to oil reservoirs by interfacial tension measurement. Colloids Surf A 466:138–146. https://doi.org/10.1016/j.colsurfa.2014.10.053
Kharrat AM, Indo K, Mostowfi F (2013) Asphaltene content measurement using an optical spectroscopy technique. Energy Fuels 27(5):2452–2457. https://doi.org/10.1021/ef400050y
Kokal S, Sayegh SG (1995) Asphaltenes: the cholesterol of petroleum. Proc Middle East Oil Show 1:169–181. https://doi.org/10.2118/29787-ms
Lashkarbolooki M, Ayatollahi S (2018) Experimental investigation on CO2-light crude oil interfacial and swelling behavior. Chin J Chem Eng 26(2):373–379. https://doi.org/10.1016/j.cjche.2017.07.010
Lashkarbolooki M, Eftekhari MJ, Najimi S, Ayatollahi S (2017) Minimum miscibility pressure of CO2 and crude oil during CO2 injection in the reservoir. J Supercrit Fluids 127:121–128. https://doi.org/10.1016/j.supflu.2017.04.005
Leontaritis KJ, Mansoori GA (1988) Asphaltene deposition: a survey of field experiences and research approaches. J Pet Sci Eng 1(3):229–239. https://doi.org/10.1016/0920-4105(88)90013-7
Liao H, Morte M, Hascakir B (2019) Effect of crude oil composition on microwave absorption of heavy oils. In: SPE western regional meeting. SPE-195263-MS. https://doi.org/10.2118/195263-MS
Maqbool T, Srikiratiwong P, Fogler HS (2011) Effect of temperature on the precipitation kinetics of asphaltenes. Energy Fuels 25(2):694–700. https://doi.org/10.1021/ef101112r
Miadonye A, Evans L (2010) The solubility of asphaltenes in different hydrocarbon liquids. Pet Sci Technol 28(14):1407–1414. https://doi.org/10.1080/10916460902936960
Mohammed I, Mahmoud M, Al Shehri D, El-Husseiny A, Alade O (2021) Asphaltene precipitation and deposition: a critical review. J Pet Sci Eng. https://doi.org/10.1016/j.petrol.2020.107956
Mullins OC (2008) Review of the molecular structure and aggregation of asphaltenes and petroleomics. SPE J 13(01):48–57. https://doi.org/10.2118/95801-PA
Orr FM, Silva MK (1987) Effect of oil composition on minimum miscibility pressure-part 2: correlation. SPE Reserv Eng 2(04):479–491. https://doi.org/10.2118/14150-PA
Orr FM, Silva MK, Li Lien C (1983) Equilibrium phase compositions of CO2/crude oil mixtures-part 2: comparison of continuous multiple-contact and slim-tube displacement tests. SPE J 23(02):281–291. https://doi.org/10.2118/10725-PA
Prakoso AA, Punase AD, Hascakir B (2017) A mechanistic understanding of asphaltenes precipitation from varying-saturate-concentration perspectives. SPE Prod Oper 32(1):86–98. https://doi.org/10.2118/177280-pa
Punase A, Prakoso A, Hascakir B (2016) The polarity of crude oil fractions affects the asphaltenes stability. In: SPE western regional meeting. SPE-180423-MS. https://doi.org/10.2118/180423-MS
Rezk MG, Foroozesh J (2019) Phase behavior and fluid interactions of a CO2—Light oil system at high pressures and temperatures. Heliyon 5(April):e02057. https://doi.org/10.1016/j.heliyon.2019.e02057
Rogel E, Ovalles C, Moir ME, Schabron JF (2009) Determination of asphaltenes in crude oil and petroleum products by the on column precipitation method. Energy Fuels 23(9):4515–4521. https://doi.org/10.1021/ef900358q
Rosiani D, Permadi AK, Siregar HPS, Gunawan AY, Ariadji T (2022) A new CO2—EOR methods screening model based on interdependency parameters. Appl Sci. https://doi.org/10.3390/app12083937
Saboor A, Yousaf N, Haneef J, Ali SI, Lalji SM (2022) Performance of asphaltene stability predicting models in field environment and development of new stability predicting model (ANJIS). J Pet Explor Prod Technol 12(5):1423–1436. https://doi.org/10.1007/s13202-021-01407-8
Santos D, Amaral M, Filho EBM, Dourado RS, Coutinho JAP, Borges GR, Franceschi E, Dariva C (2019) Revisiting the methodology for asphaltenes precipitation. J Pet Sci Eng 178:778–786. https://doi.org/10.1016/j.petrol.2019.03.074
Sarma HK (2003) Can we ignore asphaltene in a gas injection project for light-oils? In: Proceedings—SPE international improved oil recovery conference in Asia Pacific, pp 193–199. https://doi.org/10.2523/84877-ms
Siagian UWR, Grigg RB (1998) The extraction of hydrocarbons from crude oil by high pressure CO2. In: SPE/DOE improved oil recovery symposium. SPE-39684-MS. https://doi.org/10.2118/39684-MS
Soleymanzadeh A, Yousefi M, Kord S, Mohammadzadeh O (2019) A review on methods of determining onset of asphaltene precipitation. J Pet Explor Prod Technol 9(2):1375–1396. https://doi.org/10.1007/s13202-018-0533-5
Subramanian S, Simon S, Sjöblom J (2016) Asphaltene precipitation models: a review. J Dispers Sci Technol 37(7):1027–1049. https://doi.org/10.1080/01932691.2015.1065418
Sulaimon AA, De Castro JKM, Vatsa S (2020) New correlations and deposition envelopes for predicting asphaltene stability in crude oils. J Pet Sci Eng. https://doi.org/10.1016/j.petrol.2019.106782
Wang S, Chen S, Li Z (2016) Characterization of produced and residual oils in the CO2 flooding process. Energy Fuels 30(1):54–62. https://doi.org/10.1021/acs.energyfuels.5b01828
Xiong R, Guo J, Kiyingi W, Feng H, Sun T, Yang X, Li Q (2020) Method for judging the stability of asphaltenes in crude oil. ACS Omega 5(34):21420–21427. https://doi.org/10.1021/acsomega.0c01779
Yellig WF, Metcalfe RS (1980) Determination and prediction of CO2 minimum miscibility pressures. J Pet Technol 32(01):160–169. https://doi.org/10.2118/7477-PA
Zanganeh P, Ayatollahi S, Alamdari A, Zolghadr A, Dashti H, Kord S (2012) Asphaltene deposition during CO2 injection and pressure depletion: a visual study. Energy Fuels 26(2):1412–1419. https://doi.org/10.1021/ef2012744
Zhang K, Gu Y (2015) Two different technical criteria for determining the minimum miscibility pressures (MMPs) from the slim-tube and core flood tests. Fuel 161(August):146–156. https://doi.org/10.1016/j.fuel.2015.08.039
Zhang N, Wei M, Bai B (2018) Statistical and analytical review of worldwide CO2 immiscible field applications. Fuel 220(December 2017):89–100. https://doi.org/10.1016/j.fuel.2018.01.140
Zhang K, Jia N, Zeng F, Li S, Liu L (2019) A review of experimental methods for determining the Oil-Gas minimum miscibility pressures. J Pet Sci Eng 183(July):106366. https://doi.org/10.1016/j.petrol.2019.106366
Zick AA (1986) A combined condensing/vaporizing mechanism in the displacement of oil by enriched gases. In: Proceedings—SPE annual technical conference and exhibition. SPE-15493-MS. https://doi.org/10.2118/15493-ms
Funding
This work was financially supported by Indonesia Endowment Fund for Education (LPDP), Ministry of Finance of the Republic of Indonesia, through Grant No. KET-1277/LPDP.4/2019. The experiments were conducted in the Laboratory of Enhanced Oil Recovery (EOR), PERTAMINA Research and Technology Innovation (RTI), PT. PERTAMINA (Persero) Indonesia. The authors also appreciate the Universitas Trisakti and Institut Teknologi Bandung (ITB) for their encouragement provided in the writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the co-authors, the corresponding author wishes to confirm that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hartono, K.F., Permadi, A.K., Siagian, U.W.R. et al. The impacts of CO2 flooding on crude oil stability and recovery performance. J Petrol Explor Prod Technol 14, 107–123 (2024). https://doi.org/10.1007/s13202-023-01699-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01699-y