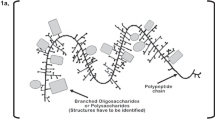
Abstract
The binding property of Con A has been studied intensively and applied widely to glycoconjugates / glycobiology for over 80 years. However, its role and functional relationship of Con A with these mammalian structural units, glycotopes, N-glycan chains, as well as their polyvalent forms in N-glycoproteins involved in the Con A-glycan interactions have not been well defined and organized. In this study, the recognition factors involved in these interactions were analyzed by our well developed method- the enzyme linked lectinosorbent (ELLSA) and inhibition assay. Based on all the data obtained, it is concluded that Con A, as previously reported, has a relatively broad and wide recognition ability of the Manα1 → and Glcα1 → related glycans. It reacted not only strongly with yeast mannan and glycogens, but also bound well with a large number of mammalian N-glycans, including the N-glycans of rat sublingual gp (RSL), human Tamm-Horsfall glycoprotein (THGP), thyroglobulin and lactoferrin. The recognition specificity of Con A towards ligands, expressed by Molar Relative Potency (Molar R.P.), in a decreasing order is as follows: α1 → 3, α1 → 6 Mannopentaose (M5) and Biantennary N-linked core pentasaccharide (MDi) ≥ α1 → 3, α1 → 6 Mannotriose (M3) > Manα1 → 3Man (α1 → 3Mannobiose), Manα1 → 2Man (α1 → 2Mannobiose), Manα1 → 6Man (α1 → 6Mannobiose), Manα1 → 4Man (α1 → 4Mannobiose) > GlcNAcβ1 → 2Man (β1 → 2 N-Acetyl glucosamine-mannose) > Manα1 → /Glcα1 → > Man > Glc, while Gal / GalNAc were inactive. Furthermore, the Man related code system, in this study, is proposed to express by both numbers of Man and GlcNAcβ1 → branches (M3 to M9 / MMono to Penta etc.) and a table of three Manα1 → and Glcα1 → related biomasses of six recognition factors involved in the Con A-glycan interactions has also been demonstrated. These themes should be one of the most valuable advances since 1980s.





Similar content being viewed by others
Availability of data and materials
It has been described in the tables of our manuscript.
Abbreviations
- C(G)BP:
-
Carbohydrate (Glycan) Binding Protein
- Con A:
-
Concanavalin A lectin
- Morniga-M:
-
Morus nigra lectin
- Lentil:
-
Lens culinaris lectin
- PSA:
-
Pea (Pisum sativum) lectin
- THGP:
-
Tamm-Horsfall glycoprotein
- HOC:
-
Cyst gps: glycoproteins isolated from human ovarian cyst fluid
- RSL:
-
Rat sublingual gp-major
- OSM:
-
Ovine submandibular gp-major
- PSM:
-
Porcine salivary mucin-major
- BSM:
-
Bovine submandibular gp-major
- Man:
-
D-manno pyranose
- Glc:
-
D-glucopyranose
- GlcNAc:
-
N-Acetylglucosamine
- Gal:
-
D-galactopyranose
- GalNAc:
-
N-Acetylgalactosamine
- GalN:
-
Galactosamine
- LFuc:
-
6-deoxy-L-galactopyranose
- Neu5Ac:
-
SA, NeuAc, sialic acid
- LacNAc:
-
N-Acetyllactosamine
- M 3 / M 5 :
-
The No. of subscript in Man indicates the No. of Man in the structures of the mammalian N-glycans
- M or M 3 :
-
Manα1 → 6(Manα1 → 3)Man, or α1 → 3, α1 → 6 Mannotriose
- M 5 :
-
Manα1 → 6(Manα1 → 3)Manα1 → 6(Manα1 → 3)Man, or α1 → 3, α1 → 6 Mannopentaose
- M Mono to Tetra :
-
The Mono to Tetra or more of subscript in Man indicates the No. of GlcNAcβ1 → or IIβ branches in the structures of the mammalian N-glycan chain
- Tn :
-
GalNAcα1 → Ser/Thr
- T :
-
Thomsen-Friedenreich disaccharide
- T α :
-
Galβ1 → 3GalNAcα1 →
- A :
-
GalNAcα1 → 3Gal
- A h :
-
GalNAcα1 → 3(LFucα1 → 2)Gal (Human blood group A glycotope)
- B :
-
Galα1 → 3Gal
- B h :
-
Galα1 → 3(LFucα1 → 2)Gal (Human blood group B glycotope)
- H :
-
LFucα1 → 2Gal (Human blood group O (H) glycotope)
- h :
-
Crypto LFucα1 → 2Gal
- I β :
-
Galβ1 → 3GlcNAcβ1 →, human blood group type I precursor sequence
- II β :
-
Galβ1 → 4GlcNAcβ, human blood group type II precursor sequence
- Le a :
-
Lewisa, Galβ1 → 3(Fucα1 → 4)GlcNAc
- Le b :
-
Lewisb, Fucα1 → 2Galβ1 → 3(Fucα1 → 4)GlcNAc
- Le x :
-
Lewisx, Galβ1 → 4(Fucα1 → 3)GlcNAc
- Le y :
-
Lewisy, Fucα1 → 2Galβ1 → 4(Fucα1 → 3)GlcNAc
- sLe a :
-
Sialyl Lewisa, NeuAcα2 → 3Galβ1 → 3(Fucα1 → 4)GlcNAc
- sLe x :
-
Sialyl Lewisx, NeuAcα2 → 3Galβ1 → 4(Fucα1 → 3)GlcNAc
- C :
-
GlcNAcβ1 → 4GlcNAc, chitin disaccharide
- C f :
-
GlcNAcβ1 → 4(LFucα1 → 6)GlcNAc
- RFs:
-
Six Recognition Factors have been selected and defined- RF-1 to RF-6 or RF-(i) to (vi)
- Molar R.P.:
-
Molar Relative Potency for the avidities and/or intensities of RF-1 to RF-5 or RF-(i) to RF-(v)
- Mass R.P.:
-
Mass Relative Potency for the avidity and/or intensity of RF-6 or RF-(vi)
- ELLSA:
-
Enzyme linked lectinosorbent assay
- ELLISA:
-
Inhibition assay
- TBS:
-
Tris-HCl buffered saline
- TBS-T:
-
TBS with Tween 20
- PBS:
-
Phosphate-buffered saline
- MMCO:
-
Molecular mass cut off
References
Goldstein, I.J., Poretz, R.D.: Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener, I. E., Sharon, N., Goldstein, I. J. (eds.) The lectins: properties, functions, and applications in biology and medicine. Chapter 2, pp. 35–244, Orlando Academic Press (1986)
Goldstein, I.J., Winter, H.C., Poretz, R.D.: Plant lectins: tools for the study of complex carbohydrates. In: Montreuil, J., Vliegenthart, J.F.G. and Schachter, H. (eds.) Glycoproteins II. New comprehensive biochemistry, Vol. 29b, Chapter 12, pp. 403–470. Elsevier Science B. V., Amsterdam (1997)
Sharon, N., Lis, H.: Specificity and affinity. In: Sharon, N., Lis, H. (eds.) Lectins 2nd Ed., Chapter 4, pp.63–103, Kluwer Academic Publishers, Dordrecht, The Netherlands (2003)
Sharon, N., Lis, H.: Lectins as Cell Recognition Molecules. Science 246, 227–234 (1989)
Wu, A.M., Sugii, S.J., Herp, A.: A Guide for Carbohydrate specificities of Lectins. In: Wu, A.M. (ed.) The Molecular Immunology of Complex Carbohydrates. Adv. Exp. Med. Biol. 228, the appendix, pp. 817–853. Plenum Press, New York and London (1988)
Wu, A.M., Song, S.C., Tsai, M.S., Herp, A.: A guide to the carbohydrate specificities of applied lectins-2 (updated in 2000). In: Wu, A.M. (ed.) The Molecular Immunology of Complex Carbohydrates-2. Adv. Exp. Med. Biol. 491, pp. 551–585. Kluwer Acadmeic/Plenum Publishers (2001)
Wu, A.M., Liu, J.H., Singh, T., Yang Z.: Recognition Roles of mammalian structural units and polyvalency in lectin-glycan interactions. In: Wu, A.M. (ed.) The Molecular Immunology of Complex Carbohydrates-3, Chapter 6, Adv. Exp. Med. Biol. 705, 99–116. Springer New York Dordrecht Heidelberg London (2011)
Wu, A.M., Wu, J.H., Singh, T., Chu, K.C., Peumans, W.J., Rougé, P., Van Damme, E.J.M.: A Novel Lectin (Morniga M) from Mulberry (Morus nigra) Bark Recognizes Oligomannosyl Residues in N-Glycans. J. Biomed. Sci. 11, 874–885 (2004)
Kaku, H., Goldstein, I.J., Van Damme, E.J.M., Peumans, W.J.: New mannose-specific lectins from garlic (Allium sativum) and ramsons (Allium ursinum) blubs. Carbohydr. Res. 229, 347–353 (1992)
Van Damme, E.J.M., Smeets, K., Van Leuven, F., Peumans, W.J.: Molecular cloning of mannose-binding lectins from Clivia miniata. Plant Mol. Biol. 24, 825–830 (1994)
Sybuya, N., Goldstein, I.J., Van Damme, E.J.M., Peumans, W.J.: Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J. Biol. Chem. 263, 728–734 (1988)
Wecksler, M., Levy, A., Jaffé, W.G.: Mitogenic effects of extracts of Canavalia ensiformis and Concanavalina A. Acta Cient. Venezo. 19(4), 154–156 (1968)
Powell, A.E., Leon, M.A.: Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp. Cell. Res. 62, 315–325 (1970)
Yamashita, U., Shirakawa, F., Nakamura, H.: Production of interleukin 1 by adult T cell leukemia (ATL) cell lines. J. Immunol. 138(10), 3284–3289 (1987)
Ree, H.J.: Lectin histochemistry of malignant tumors. II. Concanavalin A: a new histochemical marker for macrophage-histiocytes in follicular lymphoma. Cancer. 51, 1639–1646 (1983)
Oliveira, S.R.B.D., Franco, Á.X., Quaresma, M.P., de Carvalho, C.M.M., Marques, F.C.J., da Silva Pantoja, P., Mendonça, V.A., Osterne, V.J.S., Correia, J.L.A., Assreuy, A.M.S., de Souza, M.H.L.P., Nascimento, K.S., Cavada, B.S., Criddle, D.N., Soares, P.M.G.: Anti-inflammatory and anti-necrotic effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in experimental acute pancreatitis. Glycoconj. J. 39, 599–608 (2022)
Edelman, G.M., Cunningham, B.A., Reeke, G.N., Jr., Becker, J.W., Waxdal, M.J., Wang, J.L.: The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci USA. 69, 2580–2584 (1972)
Becker, J.W., Reeke, G.N., Jr., Wang, J.L.: The covalent and three-dimensional structure of concanavalin A. III. Structure of the monomer and its interactions with metals and saccharides. J. Biol. Chem. 250, 1513–1524 (1975)
Barber, B.H., Carver, J.P.: Magnetic resonance studies of concanavalin A: Conformational Changes Induced by Ca2+ and alpha-methyl-D-mannopyranoside. Can. J. 53, 371–379 (1975)
Smith, E.E., Gunja Smith, Z.H., Goldstein, I.J.: Protein-carbohydrate interaction: A turbidimeteric study of the interaction of Concanavalin A with amylopectin and glycogen and some of their enzymic and chemical degradation products. Biochem. J. 107, 715–724 (1968)
Allen, A.K., Desai, N.N., Neuberger, A.: The purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of this properties with lectins of similar specificity. Biochem. J. 155, 127–135 (1976)
Goldstein, I.J., Hollerman, C.E., and Smith, E.K.: Protein-carbohydrate interaction II. Inhibition studies on the interaction of concanavalin A with polysaccharides. Biochemistry. 4, 876–883 (1965)
Baenziger, J.U., Fiete, D.: Structural determinants of concanavalin A specificity for oligosaccharides. J. Biol. Chem. 254, 2400–2407 (1979)
Hardman, K.D., Goldstein, I.J.: The Structure and Activity of Concanavalin A. In: Atassi, M.Z. (ed.) Immunochemistry of proteins. 2, pp. 373–416. Plenum Press, New York (1977)
Debray, H., Montreuil, J.: Structural basis for the affinity of four insolubilized lectins, with a specifivity for α-d-mannose, towards various glycopeptides with the N-glycosylamine linkage and related oligosaccharides. J. Biosci. 5, 93–100 (1983)
Chervenak, M.C., Toone, E.J.: Calorimetric analysis of the binding of lectins with overlapping carbohydrate-binding ligand specificities. Biochemistry 34, 5685–5695 (1995)
Mann, D.A., Kanai, M., Maly, D.J., Kiessling, L.L.: Probing low affinity and multivalent interactions with surface plasmon resonance: ligands for Concanavalin A. J. Am. Chem. Soc. 120, 10575–10582 (1998)
Dimick, S.M., Powell, S.C., McMahon, S.A., Moothoo, D.N., Naismith, J.H., Toone, E.J.: On the meaning of affinity: Cluster glycoside effect and Concanavalin A. J. Am. Chem. Soc. 121, 10286–10296 (1999)
Barre, A., Bourne, Y., Van Damme, E.J.M., Peumans, W.J., Rougé, P.: Mannose-binding plant lectins: Different structural scaffolds for a common sugar-recognition process. Biochimie 83, 645–651 (2001)
Srinivas, O., Mitra, N., Surolia, A., Jayaraman, N.: Photoswitchable cluster glycosides as tools to probe carbohydrate-protein interaction: synthesis and lectin-binding studies of azobenzene containing multivalent sugar ligands. Glycobiology 15, 861–873 (2005)
Wu, A.M.: Polyvalency of Glycotopes and their conformational features in glycans as the most powerful recognition factors for the glycan-lectin interactions. In: Banerjee, D.K. (ed.) Glycome : the hidden code in biology, Chapter 14, pp. 287–305. Nova Science, New York (2021)
Duk, M., Lisowska, E., Wu, J.H., Wu, A.M.: The biotin/avidin-mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal. Biochem. 221, 266–272 (1994)
Lisowska E., Duk, M., Wu, A.M.: Preparation of biotinylated lectins and application in microtiter plate assays and western blotting. In: Meier, T., Fahrenholz F. (eds.) A Laboratory Guide to Biotin-Labeling in Biomolecule Analysis. Biomethods. 7, 115–129. Birkhauser Verlag Basel (1996)
Wu, A.M., Lisowska, E., Duk, M., Yang, Z.: Lectins as tools in glycoconjugate research. Glycoconj. J. 26, 899–913 (2009)
Wu, A.M., Liu, J.H.: Lectins and ELLSA as powerful tools for Glycoconjugate Recognition Analyses. Glycoconj. J. 36, 175–183 (2019)
Fournier, T., Medjoubi-N, N., Porquet, D.: Alpha-1-acid glycoprotein. Biochim. Biophys. Acta 1482, 157–171 (2000)
Clemeston, K.J.: α1-Acid glycoprotein (pp.174), Fibrinogen (pp.185) of Chapter 9; Mintreuil, J., Spik, G., Nazurier, J.: Lactotransferrins (lactoferrins) (pp.206) of Chapter 10; Hughes, R.C.: Lamiinin (pp. 535–540) of Chapter 14. In: Montreuil, J., Vliegenthart, J.F.G., and Schachter, H. (eds) Glycoproteins II, Vol 29b, Elsevier Science B. V., Amsterdam (1997)
Orczyk-Pawilowicz, M., Hirnle, L., Katnik-Prastowska, I.: Alterations of N-glycan branching and expression of sialic acid on amniotic fluid alpha-1-acid glycoprotein derived from second and third trimesters of normal and prolonged pregnancies. Clin. Chim. Acta 367, 96–92 (2006)
Fournet, B., Montreuil, J., Strecker, G., Dorland, L., Haverkamp, J., Vliegenthart, J.F.G., Binette, J.P., Schmid, K.: Determination of the primary structures of 16 asialo-carbohydrate units derived from human plasma alpha 1-acid glycoprotein by 360-MHZ 1H NMR spectroscopy and permethylation analysis. Biochemistry 17, 5206–5214 (1978)
Shiyan, S.D., Bovin, N.V.: Carbohydrate composition and immunomodulatory activity of different glycoforms of alpha1-acid glycoprotein. Glycoconj J. 14, 631–638 (1997)
Brock, J.H.: The physiology of lactoferrin. Biochem. Cell Biol. 80, 1–6 (2002)
Hughes, G.J., Reason, A.J., Savoy, L.-A., Jaton, J.-C., Frutiger-Hughes, S.: Carbohydrate moieties in human secretory component. Biochem. Biophys. Acta. 1434, 86–93 (1999)
Farnaud, S., Evans, R.W.: Lactoferrin–a multifunctional protein with antimicrobial properties. Mol Immunol. 40, 395–405 (2003)
Engel, J.: Laminins and other strange proteins. Biochemistry 31, 10643–10651 (1992)
Tamm, I., Horsfall, F.L.: Characterization and Separation of an Inhibitor of Viral Hemagglutination Present in Urine. Proc. Soc. Exp. Biol. Med. 74, 108–114 (1950)
Hard, K., Van, Z.G., Moonen, P., Kamerling, J.P., Vliegenthart, F.G.: The Ash-linked carbohydrate chains of human Tamm-Horsfall glycoprotein of one male: Novel sulfated and novel N-acetylgalactosamine-containing N-linked carbohydrate chains. Eur. J. Biochem. 209, 895–915 (1992)
Lehle, L. and Tanner, W.: Yeast mannoproteins and cell wall architecture. In: Montreuil, J., Vliegenthart, J.F.G., and Schachter, H. (eds) Glycoproteins. Vol 29a, Chapter 7, pp. 476–477, Elsevier Science B. V., Amsterdam (1997)
Yoshida, Y., Naito, E., Mizukoshi, H., Watanabe, Y., Kimura, K., Yokoi, W., Sato, T., Okumura, T., Ito, M., Sawada, H.: Side-Chain Structure of Cell Surface Polysaccharide, Mannan, Affects Hypocholesterolemic Activity of Yeast. J. Agric. Food Chem. 57, 8003–8009 (2009)
Gemmill, T.R., Trimble, R.B.: Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim. Biophys. Acta. 1426, 227–237 (1999)
Campbell, M.K., Farrell, S.O.: Carbohydrates in Chapter 16. In: Campbell, M.K., Farrell, S.O. (eds.) Biochemistry, 7th edn., pp. 470–471. Cengage Learning, Brooks/Cole (2012)
Troy, F.A. II.: Polysialylation: from bacteria to brains. Glycobiology. 2, 5–23, Review (1992) a Jennings, H.J.: Capsular Polysaccharides as Vaccine Candidates. Curr. Top. Microbiol. Immunol.150, 97-127 (1990)
Tettamanti, G., Pigman, W.: Purification and characterization of bovine and ovine submaxillary mucins. Arch Biochem. Biophys. 124, 41–45 (1968)
Herp, A., Borelli, C., Wu, A.M.: Biochemistry and lectin binding properties of mammalian salivary mucous glycoproteins, In: Wu, A.M. (ed.) The Molecular Immunology of Complex Carbohydrates. Adv. Exp. Med. Biol. 228, pp. 395–435. Kluwer Academic Publishers, Plenum Press, New York and London (1988)
Wu, A.M., Csako G, Herp, A.: Structure, biosynthesis, and function of salivary mucins. Mol. Cell. Biochem. 137(1), 39–55, Review (1994)
Wu, A.M., Herp, A., Khoo, K.H., Yu, S.Y., Yang, Z.: Glycomic mapping of O- and N-linked glycans from major rat sublingual mucin. Glycoconj. J. 25, 199–212 (2008)
Kabat, E.A.: Purification of Blood Group Substances. In: Kabat, E.A. (ed.) Blood Group Substances: Their Chemistry and Immunochemistry, Chapter 4, pp. 135–139. Academic Press, New York (1956)
Wu, A.M.: Structural concepts of the blood group A, B, H, Lea, Leb, I and i active glycoproteins purified from human ovarian cyst fluid. In: Wu, A.M. (ed.) The Molecular Immunology of Complex Carbohydrates. Adv. Exp. Med. Biol. 228, pp. 351–394. Kluwer Academic Publishers, Plenum Press, New York and London (1988)
Wu, A.M.: Human Blood Group ABH/Ii, Lea,b,x,y, and Sialyl Lea,x Glycotopes Internal Structures; and Immunochemical Roles of Human Ovarian Cyst Glycoproteins. In: Chapter 3, The Molecular Immunology of Complex Carbohydrates-3 (Wu, A.M. ed.). Adv. Exp. Med. Biol. 705, 33–52 (2011)
Wu, A.M.: Glycan structures and their recognition roles in the human blood group ABH/Ii, Lea, b, x, y and Sialyl Lea, x active cyst glycoproteins. Glycoconj. J. 36, 495–507 (2019)
Wu, A.M.: Loci and motifs of the GalNAcα1→3/O related glycotopes in the mammalian glycoconjugates and their lectin recognition roles. Glycoconj. J. 39, 633–651 (2022)
Wieruszeski, J.-M., Michalski, J.-C., Montreuil, J., Strecker, G., Peter-Katalinic, J., Egge, H., van Halbeek, H., Mutsaers, J.H.G.M., Vliegenthart, J.F.G.: Structure of the monosialyl oligosaccharides derived from salivary gland mucin glycoproteins of the Chinese swiftlet (genus Collocalia). J. Biol. Chem. 262, 6650–6657 (1987)
Kamerling J.P. and Vliegenthart J.F.G.: Hemocyanins, schemes 2 (pp.123–142) of Chapter 6. In: Montreuil, J., Vliegenthart, J.F.G., and Schachter, H. (eds) Glycoproteins II, Vol 29b, Elsevier Science B. V., Amsterdam (1997)
Yamamoto, K., Tsuji, T., Irimura, T., Osawa, T.: The Structure of Carbohydrate Unit B of Porcine Thyroglobulin. Biochem. J. 195, 701–713 (1981)
Acknowledgements
This work was supported by CGU research grant, BMRP 008, BMRP 530, and MICCs Forever Fund (in process), Kwei-San, Tao-yuan, Taiwan. The author would like to thanks Dr. Ming-Sung Tsai and Ms. Chien-Ching Wu for their immunochemical analyses, and Ms. Ko’s typing assistance.
Funding
It has been indicated at the end of our manuscript as this work was supported by CGU research grant, BMRP 008, BMRP 530, and MICCs Forever Fund (in process), Kwei-San, Tao-yuan, Taiwan.
Author information
Authors and Affiliations
Contributions
It is only one author.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Competing interests
The author has no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, A.M. Roles of the structural units, glycotopes / mammalian N-glycans for Con A-glycan interactions, their codes, and their recognition factors. Glycoconj J 40, 587–608 (2023). https://doi.org/10.1007/s10719-023-10129-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-023-10129-4