Abstract
Background and Objective
The conventional technique of general anesthesia induction during a Cesarean section involves the use of opioids only after cord clamping. We hypothesized that the use of remifentanil before cord clamping might reduce the use of maternal supplemental anesthetic agents and improve the maternal hemodynamics status and neonatal adaptation of the preterm neonate.
Methods
A phase III, double-blind, randomized, placebo-controlled, hospital-based trial enrolled parturients undergoing a Cesarean section under general anesthesia before 37 weeks of gestation. Block randomization allocated pregnant women to remifentanil or placebo. The primary outcome was the rate of newborns with Apgar scores < 7 at 5 min. Secondary outcomes were maternal hemodynamic parameters, complications of anesthetic induction, use of adjuvant anesthetic agents, neonatal respiratory distress, umbilical cord pH, and lactate levels.
Results
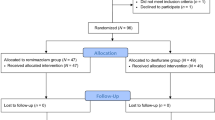
A total of 52/55 participants were analyzed, comprising 27 women in the remifentanil group and 25 in the placebo group. Nine of 27 (33.3%) neonates had an Apgar score < 7 at 5 min in the remifentanil group versus 11/25 (44.0%) in the placebo group (p = 0.45, odds ratio = 0.66, 95 confidence interval 0.20–2.18). The blood cord gases, cognitive, behavior, sensory, sleeping, and feeding scores at 1 and 2 years of corrected age were not different. For the mothers, hemodynamic parameters, anesthesia duration, and the cumulative treatment dose until cord clamping did not differ between the groups.
Conclusions
The use of a low dose of remifentanil before cord clamping for a Cesarean section appears to be safe both for the mother and the preterm newborn, but it does not improve maternal or neonatal outcomes.
Clinical Trial Registration
ClinicalTrials.gov: NCT02029898.

Similar content being viewed by others
References
Van de Velde M. The use of remifentanil during general anesthesia for caesarean section. Curr Opin Anaesthesiol. 2016;29:257–60.
Butwick AJ, El-Sayed YY, Blumenfeld YJ, Osmundson SS, Weiniger CF. Mode of anaesthesia for preterm caesarean delivery: secondary analysis from the Maternal-Fetal Medicine Units Network Caesarean Registry. Br J Anaesth. 2015;115:267–74.
Devroe S, Van de Velde M, Rex S. General anesthesia for caesarean section. Curr Opin Anaesthesiol. 2015;28:240–6.
Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016;137: e20154271.
Kamata M, Tobias JD. Remifentanil: applications in neonates. J Anesth. 2016;30:449–60.
Glass PS. Pharmacology of remifentanil. Eur J Anaesthesiol Suppl. 1995;10:73–4.
Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–74.
Ngan Kee WD, Khaw KS, Ma KC, Wong ASY, Lee BB, Ng FF. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, double-blind, controlled trial. Anesthesiology. 2006;104:14–20.
Maroni A, Aubelle M-S, Chollat C. Fetal, preterm, and term neonate exposure to remifentanil: a systematic review of efficacy and safety. Paediatr Drugs. 2023;25:537–55.
Lucas DN, Yentis SM, Kinsella SM, Holdcroft A, May AE, Wee M, et al. Urgency of caesarean section: a new classification. J R Soc Med. 2000;93:346–50.
Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol. 2000;42:816–24.
Amiel-Tison C, Gosselin J. Neurological development from birth to 6 years: user manual and examination chart. Baltimore: John Hopkins University Press; 2001.
Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91–7.
Chollat C, Bertrand E, Petit-Ledo A, de Vansay C, Voisin C, Dabaj I, et al. Cerebral palsy in very preterm infants: a nine-year prospective study in a French population-based tertiary center. J Pediatr. 2021;237: 183-9.e6.
Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–6.
Shojaei T, Wazana A, Pitrou I, Kovess V. The strengths and difficulties questionnaire: validation study in French school-aged children and cross-cultural comparisons. Soc Psychiatry Psychiatr Epidemiol. 2009;44:740–7.
Kart K, Hanci A. Effects of remifentanil and dexmedetomidine on the mother’s awareness and neonatal Apgar scores in caesarean section under general anaesthesia. J Int Med Res. 2018;46:1846–54.
Russell R. Preeclampsia and the anaesthesiologist: current management. Curr Opin Anaesthesiol. 2020;33:305–10.
Park BY, Jeong CW, Jang EA, Kim SJ, Jeong ST, Shin MH, et al. Dose-related attenuation of cardiovascular responses to tracheal intubation by intravenous remifentanil bolus in severe pre-eclamptic patients undergoing caesarean delivery. Br J Anaesth. 2011;106:82–7.
Yoo KY, Jeong CW, Park BY, Kim SJ, Jeong ST, Shin MH, et al. Effects of remifentanil on cardiovascular and bispectral index responses to endotracheal intubation in severe pre-eclamptic patients undergoing Caesarean delivery under general anaesthesia. Br J Anaesth. 2009;102:812–9.
Pournajafian A, Rokhtabnak F, Kholdbarin A, Ghodrati M, Ghavam S. Comparison of remifentanil and fentanyl regarding hemodynamic changes due to endotracheal intubation in preeclamptic parturient candidate for cesarean delivery. Anesth Pain Med. 2012;2:90–3.
Bonnet M-P, Garnier M, Keita H, Compère V, Arthuis C, Raia-Barjat T, et al. Guidelines for the management of women with severe pre-eclampsia. Anaesth Crit Care Pain Med. 2021;40: 100901.
Zhou X, Jin L-J, Hu C-Y, Chen M, Li Y, Zhang Y-S. Efficacy and safety of remifentanil for analgesia in cesarean delivery. Medicine (Baltimore). 2017;96: e8341.
White LD, Hodsdon A, An GH, Thang C, Melhuish TM, Vlok R. Induction opioids for caesarean section under general anaesthesia: a systematic review and meta-analysis of randomised controlled trials. Int J Obstet Anesth. 2019;40:4–13.
Draisci G, Valente A, Suppa E, Frassanito L, Pinto R, Meo F, et al. Remifentanil for cesarean section under general anesthesia: effects on maternal stress hormone secretion and neonatal well-being: a randomized trial. Int J Obstet Anesth. 2008;17:130–6.
Ng TKT, Cheng BCP, Chan WS, Lam KK, Chan MTV. A double-blind randomised comparison of intravenous patient-controlled remifentanil with intramuscular pethidine for labour analgesia. Anaesthesia. 2011;66:796–801.
Behdad S, Ayatollahi V, Harrazi H, Nazemian N, Heiranizadeh N, Baghianimoghadam B. Remifentanil at induction of general anesthesia for cesarean section: double blind, randomized clinical trial. Colomb Med (Cali). 2013;44:87–91.
Li C, Li Y, Wang K, Kong X. Comparative evaluation of remifentanil and dexmedetomidine in general anesthesia for cesarean delivery. Med Sci Monit. 2015;21:3806–13.
Jia Z, Li Y, Jia H, Ren J, Xie N. Curative effect of remifentanil on labor analgesia in newborns. J Matern Fetal Neonatal Med. 2020;33:1913–8.
Chevallier M, Debillon T, Pierrat V, Delorme P, Kayem G, Durox M, et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. Am J Obstet Gynecol. 2017;216:518.e1-518.e12.
Chollat C, Lecointre M, Leuillier M, Remy-Jouet I, Do Rego J-C, Abily-Donval L, et al. Beneficial effects of remifentanil against excitotoxic brain damage in newborn mice. Front Neurol. 2019;10:407.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Société Française d’Anesthésie et Réanimation and Centre Hospitalier Universitaire Rouen. The sponsors had no role in the design and conduct of the study, collection, analysis, and interpretation of result as well as the submission process.
Conflict of Interest
Clément Chollat, Fabien Tourrel, Estelle Houivet, Romain Gillet, Eric Verspyck, Maryline Lecointre, Stéphane Marret, and Vincent Compère have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
This study protocol was reviewed and approved by the Ethics Committee of Rouen, France (Comité de Protection des Personnes Nord-Ouest I, approval number 01/015/2013) and registered at ClinicalTrials.gov (NCT02029898, EudraCT 2013-001850-83, primary investigator: Fabien Tourrel, date of registration, 8 January, 2014).
Consent to Participate
Written informed consent was obtained from the participants by the research team before randomization.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Code Availability
Not applicable.
Author Contributions
CC and FT conceptualized and designed the study, coordinated and supervised the intervention, collected the data, and participated in manuscript writing and editing. EH carried out the power analyses and participated in manuscript editing. SM and RG participated in the project conception, data management, manuscript writing, and editing. EV participated in manuscript writing and editing. ML participated in the data collection, data management, and manuscript editing. VC analyzed and interpreted the data and participated in manuscript editing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chollat, C., Tourrel, F., Houivet, E. et al. Low-Dose Remifentanil in Preterm Cesarean Section with General Anesthesia: A Randomized Controlled Trial. Pediatr Drugs 26, 71–81 (2024). https://doi.org/10.1007/s40272-023-00591-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00591-w