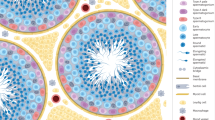
Abstract
Men’s reproductive health exclusively depends on the appropriate maturation of certain germ cells known as sperm. Certain illnesses, such as Klinefelter syndrome, cryptorchidism, and syndrome of androgen insensitivity or absence of testis maturation in men, resulting in the loss of germ cells and the removal of essential genes on the Y chromosome, can cause non-obstructive azoospermia. According to laboratory research, preserving, proliferating, differentiating, and transplanting spermatogonial stem cells or testicular tissue could be future methods for preserving the fertility of children with cancer and men with azoospermia. Therefore, new advances in stem cell research may lead to promising therapies for treating male infertility. The rate of progression and breakthrough in the area of in vitro spermatogenesis is lower than that of SSC transplantation, but newer methods are also being developed. In this regard, tissue and cell culture, supplements, and 3D scaffolds have opened new horizons in the differentiation of stem cells in vitro, which could improve the outcomes of male infertility. Various 3D methods have been developed to produce cellular aggregates and mimic the organization and function of the testis. The production of an artificial reproductive organ that supports SSCs differentiation will certainly be a main step in male infertility treatment.




Similar content being viewed by others
Data availability
Data obtained from the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- 3-LGS:
-
Three-layer gradient system
- AM:
-
Additive manufacturing
- ART:
-
Assisted reproductive technologies
- Cd-ECM:
-
Cell-derived ECM
- ECM:
-
Extracellular matrix
- EDTA:
-
Ethylenediaminetetraacetic acid
- ESCs:
-
Embryonic stem cells
- htECM:
-
Human testis ECM
- ICSI:
-
Intracytoplasmic sperm injection
- iPSCs:
-
Induced pluripotent stem cells
- NFE:
-
Near-field electrospinning
- PCL:
-
Polycaprolactone
- PEO:
-
Polyethylene oxide
- PLA:
-
Polylactic acid
- PLLA:
-
Poly L-lactic acid
- ptECM:
-
Porcine testis ECM
- PVA:
-
Polyvinyl alcohol
- SACS:
-
Soft agar culture system
- SDC:
-
Sodium deoxycholate
- SDS:
-
Sodium dodecyl sulfate
- SIS-ECM:
-
Small intestinal submucosa
- SSCs:
-
Spermatogonial stem cells
- RA:
-
Retinoic acid
- TCS:
-
Testicular cellular suspension
- TESE:
-
Testicular sperm extraction
- TT:
-
Testicular tissue
- TX-100:
-
Triton X-100
- UBM-ECM:
-
Urinary bladder matrix
References
AbuMadighem A, Shuchat S, Lunenfeld E, Yossifon G, Huleihel M (2022) Testis on a chip—a microfluidic three-dimensional culture system for the development of spermatogenesis in-vitro. Biofabrication 14(3):035004
Alves-Lopes JP, Söder O, Stukenborg J-B (2017) Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 130:76–89
Alves-Lopes JP, Söder O, Stukenborg J-B (2018) Use of a three-layer gradient system of cells for rat testicular organoid generation. Nat Protoc 13(2):248–259
Alves-Lopes JP, Stukenborg J-B (2018) Testicular organoids: a new model to study the testicular microenvironment in vitro? Hum Reprod Update 24(2):176–191
Amirkhani Z, Movahedin M, Baheiraei N, Ghiaseddin A (2022) Mini bioreactor can support in vitro spermatogenesis of mouse testicular tissue. Cell J (yakhteh) 24(5):277
Ant MP, Vanha-Perttula T (1972) Identification and enzyme quantitation of the stages of the seminiferous epithelial wave in the rat. Anat Rec 174(4):435–449
Araki Y, Sato T, Katagiri K, Kubota Y, Araki Y, Ogawa T (2010) Proliferation of mouse spermatogonial stem cells in microdrop culture. Biol Reprod 83(6):951–957
Assunção M, Dehghan-Baniani D, Yiu CHK, Später T, Beyer S, Blocki A (2020) Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front Bioeng Biotechnol 8:1378
Azizi H, Hamidabadi HG, Skutella T (2019) Differential proliferation effects after short-term cultivation of mouse spermatogonial stem cells on different feeder layers. Cell J (yakhteh) 21(2):186
Baert Y, De Kock J, Alves-Lopes JP, Söder O, Stukenborg J-B, Goossens E (2017) Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Reports 8(1):30–38
Baert Y, Dvorakova-Hortova K, Margaryan H, Goossens E (2019) Mouse in vitro spermatogenesis on alginate-based 3D bioprinted scaffolds. Biofabrication 11(3):035011
Baert Y, Stukenborg J-B, Landreh M, De Kock J, Jörnvall H, Söder O, Goossens E (2015) Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod 30(2):256–267
Bahadur G (2004) Ethics of testicular stem cell medicine. Hum Reprod 19(12):2702–2710
Barratt CL, Björndahl L, De Jonge CJ, Lamb DJ, Osorio Martini F, McLachlan R, Oates RD, van der Poel S, St John B, Sigman M, Sokol R (2017) The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance—challenges and future research opportunities. Human Reprod Upd 23(6):660–680
Bashiri Z, Amiri I, Gholipourmalekabadi M, Falak R, Asgari H, Maki CB, Moghaddaszadeh A, Koruji M (2021) Artificial testis: testicular tissue extracellular matrix as a potential bio-ink for 3D printing. Biomater Sci. https://doi.org/10.1039/D0BM02209H
Bashiri Z, Gholipourmalekabadi M, Falak R, Amiri I, Asgari H, Chauhan NP, Koruji M (2022a) In vitro production of mouse morphological sperm in artificial testis bioengineered by 3D printing of extracellular matrix. Int J Biol Macromol 217:824–841. https://doi.org/10.1016/j.ijbiomac.2022.07.127
Bashiri Z, Moghaddaszadeh A, Falak R, Khadivi F, Afzali A, Abbasi M, Sharifi AM, Asgari HR, Ghanbari F, Koruji M (2023) Generation of haploid spermatids on silk fibroin-alginate-laminin-based porous 3D scaffolds. Macromol Biosci e2200574. https://doi.org/10.1002/mabi.202200574
Bashiri Z, Zahiri M, Allahyari H, Esmaeilzade B (2022b) Proliferation of human spermatogonial stem cells on optimized PCL/Gelatin nanofibrous scaffolds. Andrologia e14380
Bhaskar R, Han SS, Gupta MK (2022) Generation of tail bearing sperm-like cells from in vitro spermatogenesis of farming goat testis. Thai J Vet Med 52(3):473–484
Boitani C, Di Persio S, Esposito V, Vicini E (2016) Spermatogonial cells: mouse, monkey and man comparison. Semin Cell Dev Biol 59:79–88. https://doi.org/10.1016/j.semcdb.2016.03.002
Bonandrini B, Figliuzzi M, Papadimou E, Morigi M, Perico N, Casiraghi F, Sangalli F, Conti S, Benigni A, Remuzzi A, Remuzzi G (2014) Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng Part A 20(9–10):1486–1498
Borges FT, Reis L, Schor N (2013) Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res 46:824–830
Bredenoord AL, Clevers H, Knoblich JA (2017) Human tissues in a dish: the research and ethical implications of organoid technology. Science 355(6322)
Brierley AS, Fernandes PG, Brandon MA, Armstrong F, Millard NW, McPhail SD, Stevenson P, Pebody M, Perrett J, Squires M (2002) Antarctic krill under sea ice: elevated abundance in a narrow band just south of ice edge. Science 295(5561):1890–1892
Cham T-C, Chen X, Honaramooz A (2021) Current progress, challenges, and future prospects of testis organoids. Biol Reprod 104(5):942–961
Cham T-C, Ibtisham F, Fayaz MA, Honaramooz A (2021) Generation of a highly biomimetic organoid, including vasculature, resembling the native immature testis tissue. Cells 10(7):1696
Champy C (1920) Quelques resultats de la methode de culture des tissus. Arch Zool Exp Gen 60:461–500
Cortez J, Leiva B, Torres CG, Parraguez VH, De los Reyes M, Carrasco A, Peralta OA (2022) Generation and characterization of bovine testicular organoids derived from primary somatic cell populations. Animals 12(17):2283
Cox B, Emili A (2006) Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc 1(4):1872–1878
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32(12):3233–3243
De Michele F, Poels J, Vermeulen M, Ambroise J, Gruson D, Guiot Y, Wyns C (2018) Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Front Physiol 9:1413
De Mori A, Peña Fernández M, Blunn G, Tozzi G, Roldo M (2018) 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 10(3):285
Dores C, Rancourt D, Dobrinski I (2015) Stirred suspension bioreactors as a novel method to enrich germ cells from pre-pubertal pig testis. Andrology 3(3):590–597
Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW (2004) Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 22(1):53–54
Dutta RC, Dutta AK (2009) Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv 27(4):334–339
Eddy E, Kahri A (1976) Cell associations and surface features in cultures of juvenile rat seminiferous tubules. Anat Rec 185(3):333–357
Edmonds ME, Woodruff TK (2020) Testicular organoid formation is a property of immature somatic cells, which self-assemble and exhibit long-term hormone-responsive endocrine function. Biofabrication 12(4):045002
Elhija MA, Lunenfeld E, Schlatt S, Huleihel M (2012) Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl 14(2):285
Eslahi N, Hadjighassem MR, Joghataei MT, Mirzapour T, Bakhtiyari M, Shakeri M, Pirhajati V, Shirinbayan P, Koruji M (2013) The effects of poly L-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int J Nanomed 8:4563
Fattahi P, Dover JT, Brown JL (2017) 3D near-field electrospinning of biomaterial microfibers with potential for blended microfiber-cell-loaded gel composite structures. Adv Healthcare Mater 6(19):1700456
Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31(4):642–648
Février B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16(4):415–421
Galuppo AG (2015) Spermatogonial stem cells as a therapeutic alternative for fertility preservation of prepubertal boys. Einstein (são Paulo) 13(4):637–639
Gao H, Cao H, Li Z, Li L, Guo Y, Chen Y, Peng G, Zeng W, Du J, Dong W (2023) Exosome-derived small RNAs in mouse Sertoli cells inhibit spermatogonial apoptosis. Theriogenology. 200:155–167. https://doi.org/10.1016/j.theriogenology.2023.02.011
Gassei K, Orwig KE (2016) Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril 105(2):256–266
Gharenaz NM, Movahedin M, Mazaheri Z (2020) Three-dimensional culture of mouse spermatogonial stem cells using a decellularised testicular scaffold. Cell J (yakhteh) 21(4):410
Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, Ogawa T (2010) In vitro murine spermatogenesis in an organ culture system. Biol Reprod 83(2):261–267
Goossens E, De Vos P, Tournaye H (2010) Array comparative genomic hybridization analysis does not show genetic alterations in spermatozoa and offspring generated after spermatogonial stem cell transplantation in the mouse. Hum Reprod 25(7):1836–1842
Goossens E, Frederickx V, De Block G, Van Steirteghem A, Tournaye H (2006a) Blastocyst development after assisted reproduction using spermatozoa obtained after testicular stem cell transplantation in mice. Hum Reprod 21(7):1759–1764
Goossens E, Frederickx V, de Block G, van Steirteghem A, Tournaye H (2006b) Evaluation of in vivo conception after testicular stem cell transplantation in a mouse model shows altered post-implantation development. Hum Reprod 21(8):2057–2060
Hamada A, Esteves SC, Nizza M, Agarwal A (2012) Unexplained male infertility: diagnosis and management. Int Braz J Urol 38(5):576–594
Hamidabadi HG, Bojnordi MN (2018) Co-culture of mouse spermatogonial stem cells with sertoli cell as a feeder layer, stimulates the proliferation and spermatogonial stemness profile. Middle East Fertility Soc J 23(2):107–111
Hammadeh M, Askari A, Georg T, Rosenbaum P, Schmidt W (1999) Effect of freeze-thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int J Andrology 22(3):155
Harris GM, Raitman I, Schwarzbauer JE (2018) Cell-derived decellularized extracellular matrices. Methods Cell Biol 143:97–114
Harrison RH, St-Pierre J-P, Stevens MM (2014) Tissue engineering and regenerative medicine: a year in review. Tissue Eng Part B Rev 20(1):1–16
Huleihel M, AbuElhija M, Lunenfeld E (2007) In vitro culture of testicular germ cells: regulatory factors and limitations. Growth Factors 25(4):236–252
Huleihel M, Lunenfeld E (2020) Approaches and technologies in male fertility preservation. Int J Mol Sci 21(15):5471
Huleihel M, Nourashrafeddin S, Plant TM (2015) Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J Androl 17(6):972
Hunter D, Anand-Ivell R, Danner S, Ivell R (2012) Models of in Vitro Spermatogenesis Spermatogenesis 2(1):32–43
Hussein KH, Park K-M, Kang K-S, Woo H-M (2016) Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater Sci Eng C 67:766–778
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326(5957):1216–1219
Ibtisham F, Awang-Junaidi AH, Honaramooz A (2020) The study and manipulation of spermatogonial stem cells using animal models. Cell Tissue Res 380(2):393–414. https://doi.org/10.1007/s00441-020-03212-x
Ibtisham F, Honaramooz A (2020) Spermatogonial stem cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells 9(3):745
Ishikura Y, Ohta H, Sato T, Murase Y, Yabuta Y, Kojima Y, Yamashiro C, Nakamura T, Yamamoto T, Ogawa T, Saitou M (2021) In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 28(12):2167–2179. https://doi.org/10.1016/j.stem.2021.08.005
Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG (2003) Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod 68(1):272–281
Jabari A, Gholami K, Khadivi F, Koruji M, Amidi F, Gilani MA, Mahabadi VP, Nikmahzar A, Salem M, Movassagh SA (2023) In vitro complete differentiation of human spermatogonial stem cells to morphologic spermatozoa using a hybrid hydrogel of agarose and laminin. Int J Biol Macromol 235:123801. https://doi.org/10.1016/j.ijbiomac.2023.123801
Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ, Nadjmi N, Khojasteh A (2017) Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater 105(2):431–459
Johnstone R, Mathew A, Mason A, Teng K (1991) Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol 147(1):27–36
Jørgensen A, Young J, Nielsen J, Joensen UN, Toft BG, Rajpert-De Meyts E, Loveland K (2014) Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. Br J Cancer 110(10):2604–2614
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M (2004) Generation of pluripotent stem cells from neonatal mouse testis. Cell 119(7):1001–1012
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T (2003) Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 69(2):612–616
Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y (2005) Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 132(18):4155–4163
Kanbar M, De Michele F, Poels J, Van Loo S, Giudice MG, Gilet T, Wyns C (2022) Microfluidic and static organotypic culture systems to support ex vivo spermatogenesis from prepubertal porcine testicular tissue: a comparative study. Front Physiol 13:884122. https://doi.org/10.3389/fphys.2022.884122
Kanninen LK, Porola P, Niklander J, Malinen MM, Corlu A, Guguen-Guillouzo C, Urtti A, Yliperttula ML, Lou YR (2016) Hepatic differentiation of human pluripotent stem cells on human liver progenitor HepaRG-derived acellular matrix. Exp Cell Res 341(2):207–217
Karlsson JO, Toner M (1996) Long-term storage of tissues by cryopreservation: critical issues. Biomaterials 17(3):243–256
Katz DJ, Kolon TF, Feldman DR, Mulhall JP (2013) Fertility preservation strategies for male patients with cancer. Nat Rev Urol 10(8):463
Kaukonen R, Jacquemet G, Hamidi H, Ivaska J (2017) Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat Protoc 12(11):2376–2390
Khadivi F, Koruji M, Akbari M, Jabari A, Talebi A, Movassagh SA, Boroujeni AP, Feizollahi N, Nikmahzar A, Pourahmadi M (2020) Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochemica 122(8):151627
Kim IG, Hwang MP, Du P, Ko J, Ha C-W, Do SH, Park K (2015) Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials 50:75–86
Kita K, Watanabe T, Ohsaka K, Hayashi H, Kubota Y, Nagashima Y, Ogawa T (2007) Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol Reprod 76(2):211–217. https://doi.org/10.1095/biolreprod.106.056895
Kobayashi H, Sato A, Otsu E, Hiura H, Tomatsu C, Utsunomiya T, Sasaki H, Yaegashi N, Arima T (2007) Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Human Mol Gen 16(21):2542–2551
Komeya M, Hayashi K, Nakamura H, Yamanaka H, Sanjo H, Kojima K, Sato T, Yao M, Kimura H, Fujii T (2017) Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci Rep 7(1):1–8
Komeya M, Kimura H, Nakamura H, Yokonishi T, Sato T, Kojima K, Hayashi K, Katagiri K, Yamanaka H, Sanjo H (2016) Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci Rep 6(1):1–10
Komeya M, Sato T, Ogawa T (2018) In vitro spermatogenesis: a century-long research journey, still half way around. Reprod Med Biol 17(4):407–420
Kumbar S, James R, Nukavarapu S, Laurencin C (2008) Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater 3(3):034002
Lacroix G, Koch W, Ritter D, Gutleb AC, Larsen ST, Loret T, Zanetti F, Constant S, Chortarea S, Rothen-Rutishauser B (2018) Air-liquid Interface in vitro models for respiratory toxicology research: consensus workshop and recommendations. Appl Vitro Toxicol 4(2):91–106
Le Magueresse-Battistoni B, Gérard N, Jégou B (1991) Pachytene spermatocytes can achieve meiotic process in vitro. Biochem Biophys Res Commun 179(2):1115–1121
Lee DR, Kaproth MT, Parks JE (2001) In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol Reprod 65(3):873–878
Lee DR, Kim KS, Yang YH, Oh HS, Lee SH, Chung TG, Cho JH, Kim HJ, Yoon TK, Cha KY (2006a) Isolation of male germ stem cell-like cells from testicular tissue of non-obstructive azoospermic patients and differentiation into haploid male germ cells in vitro. Human Rep 21(2):471–476
Lee J, Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto T, Morimoto H, Ogura A, Shinohara T (2009) Heritable imprinting defect caused by epigenetic abnormalities in mouse spermatogonial stem cells. Biol Reprod 80(3):518–527
Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC (2006b) In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 27(14):2845–2853
Lee JH, Oh JH, Lee JH, Kim MR, Min CK (2011) Evaluation of in vitro spermatogenesis using poly (D, L-lactic-co-glycolic acid)(PLGA)-based macroporous biodegradable scaffolds. J Tissue Eng Regen Med 5(2):130–137
Lee SJ, Heo DN, Park JS, Kwon SK, Lee JH, Lee JH, Kim WD, Kwon IK, Park SA (2015) Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a 3D printing system. Phys Chem Chem Phys 17(5):2996–2999
Legendre A, Froment P, Desmots S, Lecomte A, Habert R, Lemazurier E (2010) An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials 31(16):4492–4505
Lestari SW, Aditya D, Husna FA, Pratama G, Suryandari DA, Sumapradja K, Margiana R, Sari P, Kodariah R (2022) Human spermatogonia stem cells (SSCS) in a culture system with platelet rich plasma and correlations with spermatogenesis level. J Med Pharm Allied Sci 11(1):4409–4416
Li Q, Li H, Liang J, Mei J, Cao Z, Zhang L, Luo J, Tang Y, Huang R, Xia H, Zhang Q (2021) Sertoli cell-derived exosomal MicroRNA-486-5p regulates differentiation of spermatogonial stem cell through PTEN in mice. J Cell Mol Med 25(8):3950–3962
Maia FR, Reis RL, Oliveira JM (2020) Decellularized hASCs-derived matrices as biomaterials for 3D in vitro approaches. Methods Cell Biol 156:45–58
Marh J, Tres LL, Yamazaki Y, Yanagimachi R, Kierszenbaum AL (2003) Mouse round spermatids developed in vitro from preexisting spermatocytes can produce normal offspring by nuclear injection into in vivo-developed mature oocytes. Biol Reprod 69(1):169–176
Marques C, Costa P, Vaz B, Carvalho F, Fernandes S, Barros A, Sousa M (2008) Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. MHR Basic Sci Reprod Med 14(2):67–74
Martinovitch P (1938) The development in vitro of the mammalian gonad. Ovary and ovogenesis. Proc R Soc B Biol Sci 125(839):232–249
Martinovitch PN (1937) Development in vitro of the mammalian gonad. Nature 139(3514):413–413
Matsumura T, Sato T, Abe T, Sanjo H, Katagiri K, Kimura H, Fujii T, Tanaka H, Hirabayashi M, Ogawa T (2021) Rat in vitro spermatogenesis promoted by chemical supplementations and oxygen-tension control. Sci Rep 11(1):1–13
McLean DJ (2005) Spermatogonial stem cell transplantation and testicular function. Cell Tissue Res 322(1):21–31
Mincheva M, Sandhowe-Klaverkamp R, Wistuba J, Redmann K, Stukenborg JB, Kliesch S, Schlatt S (2018) Reassembly of adult human testicular cells: can testis cord-like structures be created in vitro? MHR Basic Sci Reprod Med 24(2):55–63
Mohaqiq M, Movahedin M, Mazaheri Z, Amirjannati N (2019) In vitro transplantation of spermatogonial stem cells isolated from human frozen–thawed testis tissue can induce spermatogenesis under 3-dimensional tissue culture conditions. Biol Res 52(1):16
Moore MC, Pandolfi V, McFetridge PS (2015) Novel human-derived extracellular matrix induces in vitro and in vivo vascularization and inhibits fibrosis. Biomaterials 49:37–46
Murdock MH, David S, Swinehart IT, Reing JE, Tran K, Gassei K, Orwig KE, Badylak SF (2019) Human testis extracellular matrix enhances human spermatogonial stem cell survival in vitro. Tissue Eng Part A 25(7–8):663–676
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785
Nagano M, Ryu B-Y, Brinster CJ, Avarbock MR, Brinster RL (2003) Maintenance of mouse male germ line stem cells in vitro. Biol Reprod 68(6):2207–2214
Naghieh S, Foroozmehr E, Badrossamay M, Kharaziha M (2017) Combinational processing of 3D printing and electrospinning of hierarchical poly (lactic acid)/gelatin-forsterite scaffolds as a biocomposite: mechanical and biological assessment. Mater Des 133:128–135
Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, Ito J, Kashiwazaki N, Kikuchi K (2010) Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction 139(2):331
Narimanpour Z, Bojnordi MN, Hamidabadi HG (2022) Spermatogenic differentiation of spermatogonial stem cells on three-dimensional silk nanofiber scaffold. Middle East Fertil Soc J 27(1):15
Noghani AE, Asadpour R, Saberivand A, Mazaheri Z, Rodriguez-Wallberg KA, Hamidian G (2022) Differentiation of neonate mouse spermatogonia on two-dimensional and three-dimensional culture systems supplemented with d-Serine and Dizocilpine (MK-801). Theriogenology 191:168–178
Oatley JM, Brinster RL (2008) Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 24:263–286
Oklu R, Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, Bakht SM, Yang J, Jia W, Dell’Erba V, Assawes P (2016) 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng 45:148–163. https://doi.org/10.1007/s10439-016-1612-8
Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J (2000) Active demethylation of the paternal genome in the mouse zygote. Curr Biol 10(8):475–478
Pan F, Chi L, Schlatt S (2013) Effects of nanostructures and mouse embryonic stem cells on in vitro morphogenesis of rat testicular cords. PLoS ONE 8(3):e60054
Patra T, Pathak D, Gupta MK (2021) Comparison of two culture methods during in vitro spermatogenesis of vitrified-warmed testis tissue: organ culture vs. hanging drop culture. Cryobiology 100:142–150
Pendergraft SS, Sadri-Ardekani H, Atala A, Bishop CE (2017) Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol Reprod 96(3):720–732
Perrard MH, Sereni N, Schluth-Bolard C, Blondet A, d′ Estaing SG, Plotton I, Morel-Journel N, Lejeune H, David L, Durand P (2016) Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue. Biol Reprod 95(4):89, 81–10
Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H (2015) A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Human Reprod 30(11):2463–2475
Poplinski A, Tüttelmann F, Kanber D, Horsthemke B, Gromoll J (2010) Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl 33(4):642–649
Prasad P, Kumar A, Quadri J (2017) Regenerative medicine: a new dawn for male reproductive issues. Adv Tissue Eng Regen Med Open Access 3(1):290–294
Rafeeqi T, Kaul G (2010) Carbon nanotubes as a scaffold for spermatogonial cell maintenance. J Biomed Nanotechnol 6(6):710–717
Rahbar M, Asadpour R, Azami M, Mazaheri Z, Hamali H (2022) Improving the process of spermatogenesis in azoospermic mice using spermatogonial stem cells co-cultured with epididymosomes in three-dimensional culture system. Life Sci 310:121057
Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP (1993) Relaxation of imprinted genes in human cancer. Nature 362(6422):747–749
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383
Reuter K, Ehmcke J, Stukenborg JB, Simoni M, Damm OS, Redmann K, Schlatt S, Wistuba J (2014) Reassembly of somatic cells and testicular organogenesis in vitro. Tissue Cell 46(1):86–96
Robinson M, Bedford E, Witherspoon L, Willerth SM, Flannigan R (2022) Using clinically derived human tissue to 3-dimensionally bioprint personalized testicular tubules for in vitro culturing: first report. FandS Science 3(2):130–139
Rothenberg ME, Bryhan M, Faruqi F (2008). Human and rat hepatocytes cultured on Ultra-Web™ and Ultra-Web polyamine synthetic matrices show enhanced physiologic activity when compared to collagen. Application note-corning life sciences technical monograph CLS-AN-094. 20
Ryu B-Y, Kubota H, Avarbock MR, Brinster RL (2005) Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci 102(40):14302–14307
Sá R, Neves R, Fernandes S, Alves C, Carvalho F, Silva J, Cremades N, Malheiro I, Barros A, Sousa M (2008) Cytological and expression studies and quantitative analysis of the temporal and stage-specific effects of follicle-stimulating hormone and testosterone during cocultures of the normal human seminiferous epithelium. Biol Reprod 79(5):962–975
Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F, de Rooij DG (2009) Propagation of human spermatogonial stem cells in vitro. Jama 302(19):2127–2134
Sakib S, Dores C, Rancourt D, Dobrinski I (2016) Use of stirred suspension bioreactors for male germ cell enrichment. Bioreactors in stem cell biology. Springer, pp 111–118
Sakib S, Uchida A, Valenzuela-Leon P, Yu Y, Valli-Pulaski H, Orwig K, Ungrin M, Dobrinski I (2019) Formation of organotypic testicular organoids in microwell culture. Biol Reprod 100(6):1648–1660. https://doi.org/10.1093/biolre/ioz053
Salek F, Baharara J, Shahrokhabadi KN, Amini E (2021) The guardians of germ cells; Sertoli-derived exosomes against electromagnetic field-induced oxidative stress in mouse spermatogonial stem cells. Theriogenology 173:112–122
Salem M, Feizollahi N, Jabari A, Golmohammadi MG, Shirinsokhan A, Ghanami Gashti N, Bashghareh A, Nikmahzar A, Abbasi Y, Naji M (2023) Differentiation of human spermatogonial stem cells using a human decellularized testicular scaffold supplemented by platelet-rich plasma. Artif Organs
Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T (2011a) In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471(7339):504–507
Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, Ogawa T (2011b) In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nature Comm 2(1):1–7
Sato T, Yokonishi T, Komeya M, Katagiri K, Kubota Y, Matoba S, Ogonuki N, Ogura A, Yoshida S, Ogawa T (2012) Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc Natl Acad Sci 109(42):16934–16938
Shakeri M, Kohram H, Shahverdi A, Shahneh AZ, Tavakolifar F, Pirouz M, Shahrebabak HM, Koruji M, Baharvand H (2013) Behavior of mouse spermatogonial stem-like cells on an electrospun nanofibrillar matrix. J Assist Reprod Genet 30(3):325–332
Sharma D, Ferguson M, Zhao F (2020) A step-by-step protocol for generating human fibroblast cell-derived completely biological extracellular matrix scaffolds. Methods Cell Biol 156:3–13
Silva JC, Carvalho MS, Udangawa RN, Moura CS, Cabral JM, L. da Silva C, Ferreira FC, Vashishth D, Linhardt R (2020) Extracellular matrix decorated polycaprolactone scaffolds for improved mesenchymal stem/stromal cell osteogenesis towards a patient-tailored bone tissue engineering approach. J Biomed Mater Res B Appl Biomater 108(5):2153–2166
Sofikitis N, Pappas E, Kawatani A, Baltogiannis D, Loutradis D, Kanakas N, Giannakis D, Dimitriadis F, Tsoukanelis K, Georgiou I (2005) Efforts to create an artificial testis: culture systems of male germ cells under biochemical conditions resembling the seminiferous tubular biochemical environment. Hum Reprod Update 11(3):229–259
Sousa M, Cremades N, Alves C, Silva J, Barros A (2002) Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum Reprod 17(1):161–172
Steinberger E, Steinberger A, Perloff WH (1964) Initiation of spermatogenesis in vitro. Endocrinology 74(5):788–792
Stukenborg JB, Alves-Lopes JP, Kurek M, Albalushi H, Reda A, Keros V, Töhönen V, Bjarnason R, Romerius P, Sundin M (2018) Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Human Reprod 33(9):1677–1683
Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J (2009) New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Human Reprod 15(9):521–529
Stukenborg JB, Wistuba J, Luetjens CM, Elhija MA, Huleihel M, Lunenfeld E, Gromoll J, Nieschlag E, Schlatt S (2008) Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J Androl 29(3):312–329
Sun M, Yuan Q, Niu M, Wang H, Wen L, Yao C, Hou J, Chen Z, Fu H, Zhou F, Li C (2018) Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ 25(4):749–766
Sutcliffe AG, Peters CJ, Bowdin S, Temple K, Reardon W, Wilson L, Clayton-Smith J, Brueton LA, Bannister W, Maher ER (2006) Assisted reproductive therapies and imprinting disorders—a preliminary British survey. Human Reprod 21(4):1009–1011
Suzuki S, Sato K (2003) The fertilising ability of spermatogenic cells derived from cultured mouse immature testicular tissue. Zygote 11(4):307
Szczepny A, Hogarth CA, Young J, Loveland KL (2009) Identification of Hedgehog signaling outcomes in mouse testis development using a hanging drop-culture system. Biol Reprod 80(2):258–263
Tagelenbosch RA, de Rooij DG (1993) A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutation Research/fundamental and Molecular Mechanisms of Mutagenesis 290(2):193–200
Talebi A, Gilani MA, Koruji M, Ai J, Rezaie MJ, Navid S, Salehi M, Abbasi M (2019) Colonization of mouse spermatogonial cells in modified soft agar culture system utilizing nanofibrous scaffold: a new approach. Galen Med J 8:e1319. https://doi.org/10.31661/gmj.v8i0.1319
Tanaka A, Nagayoshi M, Awata S, Mawatari Y, Tanaka I, Kusunoki H (2003) Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil Steril 79:795–801
Tesarik J, Balaban B, Isiklar A, Alatas C, Urman B, Aksoy S, Mendoza C, Greco E (2000) In-vitro spermatogenesis resumption in men with maturation arrest: relationship with in-vivo blocking stage and serum FSH. Hum Reprod 15(6):1350–1354
Toppari J, Mali P, Eerola E (1986) Rat spermatogenesis in vitro traced by quantitative flow cytometry. J Histochem Cytochem 34(8):1029–1035
Topraggaleh TR, Valojerdi MR, Montazeri L, Baharvand H (2019) A testis-derived macroporous 3D scaffold as a platform for the generation of mouse testicular organoids. Biomaterials Science 7(4):1422–1436
Tradewell MB, Masterson TA (2020) Nonobstructive azoospermia: a spectrum, not a single disease. Fertil Steril 115(2):315. https://doi.org/10.1016/j.fertnstert.2020.09.130
Trowell O (1959) The culture of mature organs in a synthetic medium. Exp Cell Res 16(1):118–147
Tüttelmann F, Ruckert C, Röpke A (2018) Disorders of spermatogenesis: Perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med Genet 30(1):12–20
Van Saen D, Goossens E, Bourgain C, Ferster A, Tournaye H (2011) Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum Reprod 26(2):282–293
Vermeulen M, Del Vento F, De Michele F, Poels J, Wyns C (2018) Development of a cytocompatible scaffold from pig immature testicular tissue allowing human sertoli cell attachment, proliferation and functionality. Int J Mol Sci 19(1):227
Vermeulen M, Del Vento F, Kanbar M, Pyr dit Ruys S, Vertommen D, Poels J, Wyns C(2019) Generation of organized porcine testicular organoids in solubilized hydrogels from decellularized extracellular matrix. Int J Mol Sci 20(21):5476
Vij SC, Gilligan T (2016) Chemotherapy and fertility. Cancer and Fertility. Humana Press, pp 97–107
Wang D, Hildorf S, Ntemou E, Mamsen LS, Dong L, Pors SE, Fedder J, Clasen-Linde E, Cortes D, Thorup J (2022) Organotypic culture of testicular tissue from infant boys with cryptorchidism. Int J Mol Sci 23(14):7975
Weinbauer GF, Luetjens CM, Simoni M, Nieschlag E (2010) Physiology of testicular function. Andrology. Springer, pp 11–59
West JL, Hubbell JA (1999) Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 32(1):241–244
Wyns C, Van Langendonckt A, Wese F-X, Donnez J, Curaba M (2008) Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod 23(11):2402–2414
Xu CC, Chan RW, Tirunagari N (2007) A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng 13(3):551–566
Yang L, Jiang Z, Zhou L, Zhao K, Ma X, Cheng G (2017) Hydrophilic cell-derived extracellular matrix as a niche to promote adhesion and differentiation of neural progenitor cells. RSC Adv 7(72):45587–45594
Yang Y, Lin Q, Zhou C, Li Q, Li Z, Cao Z, Liang J, Li H, Mei J, Zhang Q, Xiang Q (2020) A testis-derived hydrogel as an efficient feeder-free culture platform to promote mouse spermatogonial stem cell proliferation and differentiation. Front Cell Dev Biol 8:250. https://doi.org/10.3389/fcell.2020.00250
Yokonishi T, Sato T, Katagiri K, Komeya M, Kubota Y, Ogawa T (2013) In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol Reprod 89(1):15, 11–16
Yoshimoto H, Shin Y, Terai H, Vacanti J (2003) A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 24(12):2077–2082
Yuan Y, Li L, Cheng Q, Diao F, Zeng Q, Yang X, Wu Y, Zhang H, Huang M, Chen J (2020) In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res 30(3):244–255
Zhang J, Hatakeyama J, Eto K, Abe S-I (2014) Reconstruction of a seminiferous tubule-like structure in a 3 dimensional culture system of re-aggregated mouse neonatal testicular cells within a collagen matrix. Gen Comp Endocrinol 205:121–132
Zhang W, Yang J, Zhu Y, Sun X, Guo W, Liu X, Jing X, Guo G, Guo Q, Peng J (2019) Extracellular matrix derived by human umbilical cord-deposited mesenchymal stem cells accelerates chondrocyte proliferation and differentiation potential in vitro. Cell Tissue Bank 20(3):351–365
Zhang X, Wang L, Zhang X, Ren L, Shi W, Tian Y, Zhu J, Zhang T (2017) The use of KnockOut serum replacement (KSR) in three dimensional rat testicular cells co-culture model: an improved male reproductive toxicity testing system. Food Chem Toxicol 106:487–495
Ziloochi Kashani M, Bagher Z, Asgari HR, Najafi M, Koruji M, Mehraein F (2020) Differentiation of neonate mouse spermatogonial stem cells on three-dimensional agar/polyvinyl alcohol nanofiber scaffold. Syst Biol Reprod Med 66(3):202–215
Acknowledgements
We thank Dr. Ali Honaramooz for her critical review of the first draft of the manuscript. The authors also thank Dr. SeyedJamal Hoseini for editing the manuscript.
Funding
This study was funded by a grant from Iran National Science Foundation (INSF, 4004177) and Iran University of Medical Sciences (IUMS, 1400-1178).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bashiri, Z., Gholipourmalekabadi, M., Khadivi, F. et al. In vitro spermatogenesis in artificial testis: current knowledge and clinical implications for male infertility. Cell Tissue Res 394, 393–421 (2023). https://doi.org/10.1007/s00441-023-03824-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03824-z