Abstract
Background
5-Fluorouracil (5-FU) is a good anti-cancer drug, but its prolonged use results in overexpression of certain enzymes like thymidylate synthase and causes multidrug resistance in cancer cells. Many studies proven that a combination of curcumin (CUR) and 5-FU can overcome the above difficulties. It was in this direction that a novel material that could reversibly bind with drug molecules was designed and developed to overcome drug resistance.
Methods
ZIF-8 was surface modified with nitro groups (NZIF-8); 5-FU was encapsulated in the NZIF-8 through one pot method. Nitro groups on the surface of NZIF-8 enable coating of curcumin loaded chitosan, thereby forming CUR-loaded chitosan-coated 5-FU encapsulated NZIF-8 (CUR-loaded CS-coated 5-FU@NZIF-8). The drug loading and in vitro drug release studies were conducted using UV–Visible spectrophotometry at 266 nm for 5-FU and 425 nm for CUR, respectively.
Results
The nanocarrier exhibited an average size of 60 nm and was highly stable as indicated by thermogravimetric studies. The residual positive charge imparted by the amino groups on chitosan enabled enhanced cellular uptake and maybe responsible for the enhanced cytotoxicity of 5-FU + CUR combination in MCF-7 breast cancer cells (83.2%) compared to that of individual drug treatment without the nanocarrier (67.7%).
Conclusion
The study demonstrates the capability of CUR-loaded CS-coated 5-FU@NZIF-8 for combination therapy to overcome drug resistance. The results obtained from MTT assay suggest the potential applicability of the present material in the sustained delivery of simultaneous drugs. This approach can further improve efficacy as well as the cost effectiveness of chemotherapy.
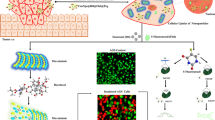
Graphical Abstract
Schematic representation of combination therapy using CUR-loaded CS-coated 5-FU@NZIF-8
Highlights
-
A dual drug delivery system was used in which CUR-loaded chitosan-coated 5-FU encapsulated nitro modified ZIF-8 was synthesized for combination therapy.
-
The surface of ZIF-8 was modified with nitro groups which enables effective coating of chitosan.
-
In a tumor environment, which is acidic in nature, chitosan swells thereby CUR releases quickly, while due to chitosan coating the encapsulated 5-FU releases slowly.
-
The positively charged amino group on the surface of chitosan helps for targeted delivery since tumor cell surface is negatively charged.











Similar content being viewed by others
References
Claessens AK, Ibragimova KI, Geurts SM, Bos ME, Erd- kamp FL, Tjan-Heijnen VC. The role of chemotherapy in treatment of advanced breast cancer: an overview for clinical practice, Critical Reviews in Oncology/Hematology. 2020;153: 102988.
Abotaleb M, Kubatka P, Caprnda M, Varghese E, Zolakova B, Zubor P, Opatrilova R, Kruzliak P, Stefanicka P, Bu¨sselberg D. Chemotherapeutic agents for the treatment of metastatic breast cancer: an update. Biomed Pharmacother. 2018;101: 458–477.
Nedeljkovi´c M, Damjanovi´c A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cells. 2019;8(9): 957.
Januˇskeviˇciene˙ I, Petrikaite˙ V. Heterogeneity of breast cancer: the importance of interaction between different tumor cell populations. Life Sci. 2019;239: 117009.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
Gote V, Nookala AR, Bolla PK, Pal D. Drug resistance in metastatic breast cancer: tumor targeted nanomedicine to the rescue. Int J Mol Sci. 2021;22(9):4673.
Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. The Breast. 2015;24:S149–53.
Chan J, Adderley H, Alameddine M, Armstrong A, Arundell D, Fox R, Harries M, Lim J, Salih Z, Tetlow C, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres. Eur J Cancer Care. 2021;30(3): e13395.
Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer. Drugs. 2003;63(15):1549–63.
Meng X, Jia K, Sun K, Zhang L, Wang Z. Smart responsive nanoplatform via in situ forming disulfiram-copper ion chelation complex for cancer combination chemotherapy. Chem Eng J. 2021;415: 128947.
Zhang Q, Lu Q-B. New combination chemotherapy of cisplatin with an electron-donating compound for treatment of multiple cancers. Sci Rep. 2021;11(1):1–13.
Pomeroy AE, Schmidt EV, Sorger PK, Palmer AC. Drug independence and the curability of cancer by combination chemotherapy, Trends in Cancer. 2022.
Zheng X, Yang X, Lin J, Song F, Shao Y. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine. 2021;85: 153547.
Sreekanth C, Bava S, Sreekumar E, Anto R. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene. 2011;30(28):3139–52.
Thulasidasan AKT, Retnakumari AP, 0Shankar M, Vijayaku- rup V, Anwar S, Thankachan S, Pillai KS, Pillai JJ, Nandan CD, Alex VV, et al. Folic acid conjugation improves the bioavailability and chemosensitizing efficacy of curcumin-encapsulated plga-peg nanoparticles towards paclitaxel chemotherapy. Oncotarget. 2017;8(64): 107374.
Leclercq IA, Farrell GC, Sempoux C, dela Pen˜a A, Horsmans Y. Curcumin inhibits nf-κb activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004;41(6): 926–934.
Huang Y-F, Zhu D-J, Chen X-W, Chen Q-K, Luo Z-T, Liu C-C, Wang G-X, Zhang W-J, Liao N-Z. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget. 2017;8(25):40264.
Sharma M, Manoharlal R, Shukla S, Puri N, Prasad T, Am- budkar SV, Prasad R. Curcumin modulates efflux mediated by yeast abc multidrug transporters and is synergistic with antifungals. Antimicrob Agents Chemother. 2009;53(8): 3256–3265.
Kalındemirta ̧s FD, Kariper IA, Sert E, Oksak N, Kuruca SE. The evaluation of anticancer activity by synthesizing 5fu loaded albumin nanoparticles by exposure to UV light. Toxicol Vitro. 2022;84: 105435.
Sethy C, Kundu CN. 5-fluorouracil (5-fu) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed Pharmacother. 2021;137: 111285.
Vinod B, Antony J, Nair H, Puliyappadamba V, Saikia M, Shyam Narayanan S, Bevin A, John Anto R. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4(2): e505–e505.
Ferguson JE, Orlando RA. Curcumin reduces cytotoxicity of 5- fluorouracil treatment in human breast cancer cells. J Med Food. 2015;18(4):497–502.
Li C, Wang Y, Du Y, Qian M, Jiang H, Wang J, Murthy N, Huang R. Side effects-avoided theranostics achieved by biodegradable magnetic silica-sealed mesoporous polymer-drug with ultralow leakage. Biomaterials. 2018;186:1–7.
Gadipelli S, Travis W, Zhou W, Guo Z. A thermally derived and optimized structure from zif-8 with giant enhancement in co 2 uptake. Energy Environ Sci. 2014;7(7):2232–8.
Park KS, Ni Z, Cˆot´e AP, Choi JY, Huang R, Uribe- Romo FJ, Chae HK, O’Keeffe M, Yaghi OM. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc Nat Acad Sci. 2006;103(27): 10186–10191.
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173(4):677–702.
Liu W, Pan Y, Xiao W, Xu H, Liu D, Ren F, Peng X, Liu J. Recent developments on zinc (ii) metal–organic framework nanocarriers for physiological pH-responsive drug delivery. MedChemComm. 2019;10(12):2038–51.
Lin W, Hu Q, Yu J, Jiang K, Yang Y, Xiang S, Cui Y, Yang Y, Wang Z, Qian G. Low cytotoxic metal–organic frameworks as temperature-responsive drug carriers. ChemPlusChem. 2016;81(8):804–10.
Wu Y-N, Zhou M, Li S, Li Z, Li J, Wu B, Li G, Li F, Guan X. Magnetic metal–organic frameworks: γ-fe2o3@ mofs via confined in situ pyrolysis method for drug delivery. Small. 2014;10(14):2927–36.
Sharsheeva A, Iglin VA, Nesterov PV, Kuchur OA, Gariful- lina E, Hey-Hawkins E, Ulasevich SA, Skorb EV, Vinogradov AV, Morozov MI. Light-controllable systems based on tio 2-zif-8 composites for targeted drug release: communicating with tumour cells. J Mater Chem B. 2019;7(43): 6810–6821.
Venna SR, Jasinski JB, Carreon MA. Structural evolution of zeolitic imidazolate framework-8. J Am Chem Soc 2010;132(51):18030–3.
Feng S, Zhang X, Shi D, Wang Z. Zeolitic imidazolate framework-8 (zif-8) for drug delivery: a critical review. Front Chem Sci Eng. 2021;15(2):221–37.
Keskin S, Kızılel S. Biomedical applications of metal organic frameworks. Ind Eng Chem Res. 2011;50(4):1799–812.
Li´edana N, Galve A, Rubio C, Tellez C, Coronas J. Caf@ zif-8: one- step encapsulation of caffeine in mof. ACS Appl Mater Interf. 2012;4(9) 5016–5021.
Zhuang J, Kuo C-H, Chou L-Y, Liu D-Y, Weerapana E, Tsung C-K. Optimized metal–organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–9.
Zheng M, Liu S, Guan X, Xie Z. One-step synthesis of nanoscale zeolitic imidazolate frameworks with high curcumin loading for treatment of cervical cancer. ACS Appl Mater Interf. 2015;7(40):22181–7.
Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62(1):28–41.
Sanchez-Salvador JL, Balea A, Monte MC, Negro C, Blanco A. Chitosan grafted/cross-linked with biodegradable polymers: a review. Int J Biol Macromol. 2021;178:325–43.
Liu X, Zhou H, Yu W, Xiong X, Krastev R, Ma X. Preparation of cationic amphiphilic nanoparticles with modified chitosan derivatives for doxorubicin delivery. Materials. 2021;14(22):7010.
Anush S, Vishalakshi B, Kalluraya B, Manju N. Synthesis of pyrazole-based Schiff bases of chitosan: evaluation of antimicrobial activity. Int J Biol Macromol. 2018;119:446–52.
Gaabour LH. Influence of silica nanoparticles incorporated with chitosan/polyacrylamide polymer nanocomposites. J Market Res. 2019;8(2):2157–63.
Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nystrom AM, Zou X. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138(3):962–8.
Jian M, Liu B, Liu R, Qu J, Wang H, Zhang X. Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv. 2015;5(60):48433–41.
Sun L, Chen Y, Zhou Y, Guo D, Fan Y, Guo F, Zheng Y, Chen W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J Pharm Sci. 2017;12(5):418–23.
Cao X-X, Liu S-L, Lu J-S, Zhang Z-W, Wang G, Chen Q, Lin N. Chitosan coated biocompatible zeolitic imidazolate framework zif-90 for targeted delivery of anticancer drug methotrexate. J Solid State Chem. 2021;300: 122259.
de Moura Ferraz LR, Tabosa AEGA, da Silva Nascimento DDS, Ferreira AS, Sales VDAW, Silva JYR, Ju´nior SA, Rolim LA, de Souza Pereira JJ, Rolim-Neto PJ. Zif-8 as a promising drug delivery system for benznidazole: development, characterization, in vitro dialysis release and cytotoxicity. Sci Rep. 2020;10(1): 1–14.
Som A, Bloch S, Ippolito JE, Achilefu S. Acidic extracellular pH of tumors induces octamer-binding transcription factor 4 expression in murine fibroblasts in vitro and in vivo. Sci Rep. 2016;6(1):1–6.
Tiwari A, Singh A, Garg N, Randhawa JK. Curcumin encapsulated zeolitic imidazolate frameworks as stimuli responsive drug delivery system and their interaction with biomimetic environment. Scientific re- ports. 2017;7(1):1–12.
Gross AF, Sherman E, Vajo JJ. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012;41(18):5458–60.
Lee Y-R, Jang M-S, Cho H-Y, Kwon H-J, Kim S, Ahn W-S. Zif- 8: a comparison of synthesis methods. Chem Eng J. 2015;271:276–80.
Vahed TA, Naimi-Jamal MR, Panahi L. Alginate-coated zif-8 metal-organic framework as a green and bioactive platform for controlled drug release. Journal of Drug Delivery Science and Technology. 2019;49:570–6.
Hu Y, Kazemian H, Rohani S, Huang Y, Song Y. In situ high pressure study of zif-8 by FTIR spectroscopy. Chem Commun. 2011;47(47):12694–6.
Maria M, Ikhmal W, Sabri M, Adnan A, et al. Identification of functional group present in Andrographis paniculata (kalmegh) leaves by FTIR analysis, in: IOP Conference Series. Mater Sci Eng. 2018;440:p. 012035.
Kevadiya BD, Patel TA, Jhala DD, Thumbar RP, Brahmb- hatt H, Pandya MP, Rajkumar S, Jena PK, Joshi GV, Gad- hia PK, et al. Layered inorganic nanocomposites: a promising carrier for 5-fluorouracil (5-fu). Eur J Pharm Biopharm. 2012;81(1): 91–101.
Feng R, Song Z, Zhai G. Preparation and in vivo pharmacokinetics of curcumin-loaded pcl-peg-pcl triblock copolymeric nanoparticles. Int J Nanomed. 2012;7:4089.
Qiu B, Xu X-F, Deng R-H, Xia G-Q, Shang X-F, Zhou P-H. Construction of chitosan/ZnO nanocomposite film by in situ precipitation. Int J Biol Macromol. 2019;122:82–7.
Shankar S, Rhim J-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocolloids. 2018;82:116–23.
Rejinold NS, Muthunarayanan M, Chennazhi K, Nair S, Jayakumar R. 5-Fluorouracil loaded fibrinogen nanoparticles for cancer drug delivery applications. Int J Biol Macromol. 2011;48(1):98–105.
Sun Q, Bi H, Wang Z, Li C, Wang X, Xu J, Zhu H, Zhao R, He F, Gai S, et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials. 2019;223: 119473.
Liang Z, Yang Z, Yuan H, Wang C, Qi J, Liu K, Cao R, Zheng H. A protein@ metal–organic framework nanocomposite for pH-triggered anticancer drug delivery. Dalton Trans. 2018;47(30):10223–8.
Ambroz F, Macdonald TJ, Martis V, Parkin IP. Evaluation of the bet theory for the characterization of meso and microporous MOFs. Small methods. 2018;2(11):1800173.
Thommes M, Kaneko K, Neimark A, Olivier J, Rodriguez-Reinoso F, Rouquerol J, Sing K. Pure Appl Chem. (2015).
Tanaka S, Kida K, Okita M, Ito Y, Miyake Y. Size-controlled synthesis of zeolitic imidazolate framework-8 (zif-8) crystals in an aqueous system at room temperature. Chem Lett. 2012;41(10):1337–9.
Popat A, Liu J, Lu GQM, Qiao SZ. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J Mater Chem. 2012;22(22):11173–8.
Crandall BS, Zhang J, Stavila V, Allendorf MD, Li Z. Desulfu- rization of liquid hydrocarbon fuels with microporous and mesoporous materials: metal-organic frameworks, zeolites, and mesoporous silicas. Ind Eng Chem Res. 2019;58(42):19322–52.
Mu L, Liu B, Liu H, Yang Y, Sun C, Chen G. A novel method to improve the gas storage capacity of zif-8. J Mater Chem. 2012;22(24):12246–52.
Cravillon J, Munzer S, Lohmeier S-J, Feldhoff A, Huber K, Wiebcke M. Rapid room-temperature synthesis and characterization of nanocrystals of a prototypical zeolitic imidazolate framework. Chem Mater. 2009;21(8):1410–2.
Gonz´alez V, Guerrero C, Ortiz U. Chemical structure and compatibility of polyamide–chitin and chitosan blends. J Appl Polym Sci. 2000;78(4): 850–857.
Yeh J-T, Chen C-L, Huang K, Nien Y, Chen J, Huang P. Synthesis, characterization, and application of pvp/chitosan blended polymers. J Appl Polym Sci. 2006;101(2):885–91.
Sankararamakrishnan N, Sanghi R. Preparation and characterization of a novel xanthated chitosan. Carbohyd Polym. 2006;66(2):160–7.
Chen Z, Xia Y, Liao S, Huang Y, Li Y, He Y, Tong Z, Li B. Thermal degradation kinetics study of curcumin with nonlinear methods. Food Chem. 2014;155:81–6.
Kariper IA, Hepokur C, Danı ̧sman-Kalındemirta ̧s F, Kuruca SE. A new method for synthesis of carbon nanoparticle and its applications. J Taibah Univ Sci. 2022;16(1): 966–975.
Khan MA, Zafaryab M, Mehdi SH, Ahmad I, Rizvi MMA. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int J Biol Macromol. 2016;93:242–53.
Abazari R, Mahjoub AR, Ataei F, Morsali A, Carpenter- Warren CL, Mehdizadeh K, Slawin AM. Chitosan immobilization on bio-MOF nanostructures: a biocompatible pH-responsive nanocarrier for doxorubicin release on mcf-7 cell lines of human breast cancer. Inorg Chem. 2018;57(21): 13364–13379.
Mazloom-Jalali A, Shariatinia Z, Tamai IA, Pakzad S-R, Malakootikhah J. Fabrication of chitosan–polyethylene glycol nanocomposite films containing zif-8 nanoparticles for application as wound dressing materials. Int J Biol Macromol. 2020;153:421–32.
Chowdhuri AR, Das B, Kumar A, Tripathy S, Roy S, Sahu SK. One-pot synthesis of multifunctional nanoscale metal-organic frameworks as an effective antibacterial agent against multidrug-resistant staphylococcus aureus. Nanotechnology. 2017;28(9): 095102.
Zhang H, Jiang W, Liu R, Zhang J, Zhang D, Li Z, Luan Y. Rational design of MOF nanocarrier-based co-delivery system of doxorubicin hydrochloride/verapamil hydrochloride for overcoming multidrug resistance with efficient targeted cancer therapy. ACS Appl Mater Interfaces. 2017;9:19687–97.
Dani ̧sman-Kalindemirta ̧s F, Ozerkan D, Kariper IA, Bulut H. The novel 5-fluorouracil loaded ruthenium-based nanocarriers enhanced anticancer and apoptotic efficiency while reducing multidrug resistance in colorectal cancer cells. J Fluoresc. 2023;33(3): 1227–1236.
Anitha A, Deepa N, Chennazhi K, Lakshmanan VK, Jayakumar R. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochimica et Biophysica Acta (BBA)-Gen Subj. 2014;1840(9): 2730–2743.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Radhakrishnan, J., Suma, S., Nair, A. et al. Curcumin-Loaded Chitosan-Coated 5-Fluorouracil Encapsulated Nanozeolitic Imidazolate Framework for Combination Cancer Therapy. J Pharm Innov 18, 2043–2053 (2023). https://doi.org/10.1007/s12247-023-09770-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09770-1