Abstract
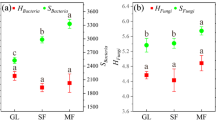
In this study, we investigated the seasonal changes in enzyme activities and microbial necromass carbon (C) in the bulk and rhizosphere soils of different halophytes (shrubs, grasses, and forbs) in an inland saline ecosystem in China. We found that the soil enzyme activities and microbial necromass C were 45–646% higher in rhizosphere soils than bulk soils regardless of the season and halophyte functional group. The activities of soil enzymes and bacterial necromass C were highest for forbs (4.9–172.3 nmol g−1 h−1 and 473.2 mg kg−1, respectively), and grasses had the highest fungal necromass C (FN-C) and total necromass C/soil organic C (TN-C/SOC) ratio. Moreover, the soil enzyme activities were higher in the late season stage (4.0–154.0 nmol g−1 h−1) than the early (3.6–132.1 nmol g−1 h−1) and middle (3.8–128.9 nmol g−1 h−1) season stages. FN-C and TN-C/SOC ratio were significantly higher in the middle season than the early and late seasons. Redundancy analysis showed that the enzyme activities and microbial necromass C mainly depended on the soil physicochemical properties, season, and halophyte functional group. Our results indicate that microbial turnover was consistently faster in rhizosphere soil than bulk soil in an inland saline ecosystem, and forbs could be used as pioneer species to improve microbial turnover during the phytoremediation of saline ecosystems.




Similar content being viewed by others
Data Availability
Data are available from the corresponding author upon reasonable request.
Change history
16 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00374-023-01771-z
References
Amelung W, Brodowski S, Sandhage-Hofmann A, Bol R (2008) Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv Agron 100:155–250
Appuhn A, Joergensen RG (2006) Microbial colonisation of roots as a function of plant species. Soil Biol Biochem 38:1040–1051
Appuhn A, Scheller E, Joergensen RG (2006) Relationships between microbial indices in roots and silt loam soils forming a gradient in soil organic matter. Soil Biol Biochem 38:2557–2564
Banfield CC, Dippold MA, Pausch J, Hoang DTT, Kuzyakov Y (2017) Biopore history determines the microbial community composition in subsoil hotspots. Biol Fert Soils 53:573–588
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Basirat M, Mousavi SM, Abbaszadeh S, Ebrahimi M, Zarebanadkouki M (2019) The rhizosheath: a potential root trait helping plants to tolerate drought stress. Plant Soil 445:565–575
Bharti P, Singh B, Bauddh K, Dey RK, Korstad J (2017) Efficiency of bioenergy plant in phytoremediation of saline and sodic soil. In: Bauddh K, Singh B, Korstad J (eds) Phytoremediation potential of bioenergy plants. Springer, Singapore, pp 353–369
Cao D, Shi F, Koike T, Lu Z, Sun J (2014) Halophyte plant communities affecting enzyme activity and microbes in saline soils of the Yellow River Delta in China. Clean-Soil Air Water 42:1433–1440
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. P Natl Acad Sci USA 104:4990–4995
Chaudhary DR, Gautam RK, Yousuf B, Mishra A, Jha B (2015) Nutrients, microbial community structure and functional gene abundance of rhizosphere and bulk soils of halophytes. Appl Soil Ecol 91:16–26
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Global Change Biol 20:2356–2367
Chen X, Hu Y, Xia Y, Zheng S, Ma C, Rui Y, He H, Huang D, Zhang Z, Ge T, Wu J, Guggenberger G, Kuzyakov Y, Su Y (2021) Contrasting pathways of carbon sequestration in paddy and upland soils. Global Change Biol 27:2478–2490
Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera CM, Rea E (2008) Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol Fert Soils 44:501–509
Crowther TW, Sokol NW, Oldfield EE, Maynard DS, Thomas SM, Bradford MA (2015) Environmental stress response limits microbial necromass contributions to soil organic carbon. Soil Biol Biochem 85:153–161
de Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29:795–811
de Vries FT, Griffiths RI, Knight CG, Nicolitch O, Williams A (2020) Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 368:270–274
Ding X, Chen S, Zhang B, Liang C, He H, Horwath WR (2019) Warming increases microbial residue contribution to soil organic carbon in an alpine meadow. Soil Biol Biochem 135:13–19
Ding X, Zhang B, Chen Q, He H, Horwath WR, Zhang X (2021) Grassland conversion to cropland decreased microbial assimilation of mineral N into their residues in a Chernozem soil. Biol Fert Soils 57:913–924
Engelking B, Flessa H, Joergensen RG (2007) Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol Biochem 39:2111–2118
Fanin N, Moorhead D, Bertrand I (2016) Eco-enzymatic stoichiometry and enzymatic vectors reveal differential C, N, P dynamics in decaying litter along a land-use gradient. Biogeochemistry 129:21–36
FAO, ITPS (2015) Status of the World’s Soil Resources (SWSR) – main report Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils. Rome, Italy
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Global Change Biol 21:2082–2094
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
González G, Seastedt TR (2001) Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 82:955–964
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787
Hannula S, Morrien E, van der Putten W, de Boer W (2020) Rhizosphere fungi actively assimilating plant-derived carbon in a grassland soil. Fungal Ecol 48:100988
Hartley IP, Garnett MH, Sommerkorn M, Hopkins DW, Fletcher BJ, Sloan VL, Phoenix GK, Wookey PA (2012) A potential loss of carbon associated with greater plant growth in the European Arctic. Nat Clim Change 2:875–879
Hartnett DC, Wilson GWT, Ott JP, Setshogo M (2013) Variation in root system traits among African semi-arid savanna grasses: implications for drought tolerance. Austral Ecol 38:383–392
He L, Mazza Rodrigues JL, Soudzilovskaia NA, Barceló M, Olsson PA, Song C, Tedersoo L, Yuan F, Yuan F, Lipson DA, Xu X (2020) Global biogeography of fungal and bacterial biomass carbon in topsoil. Soil Biol Biochem 151:108024
He M, Fang K, Chen L, Feng X, Qin S, Kou D, He H, Liang C, Yang Y (2022) Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands. Global Change Biol 28:936–949
Henneron L, Cros C, Picon-Cochard C, Rahimian V, Fontaine S (2020a) Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J Ecol 108:528–545
Henneron L, Kardol P, Wardle DA, Cros C, Fontaine S (2020b) Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol 228:1269–1282
Hernandez DJ, David AS, Menges ES, Searcy CA, Afkhami ME (2021) Environmental stress destabilizes microbial networks. ISME J 15:1722–1734
Hestrin R, Kan M, Lafler M, Wollard J, Kimbrel JA, Ray P, Blazewicz SJ, Stuart R, Craven K, Firestone M, Nuccio EE, Pett-Ridge J (2022) Plant-associated fungi support bacterial resilience following water limitation. ISME J 16:2752–2762
Hu J, Du M, Chen J, Tie L, Zhou S, Buckeridge KM, Cornelissen JHC, Huang C, Kuzyakov Y (2023) Microbial necromass under global change and implications for soil organic matter. Global Change Biol 29:3503–3515
Huang Y, Liang C, Duan X, Chen H, Li D (2019) Variation of microbial residue contribution to soil organic carbon sequestration following land use change in a subtropical karst region. Geoderma 353:340–346
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analysis. Soil Biol Biochem 111:78–84
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps, World Soil Resources Reports No 106. Rome, Italy
Ivushkin K, Bartholomeus H, Bregt AK, Pulatov A, Kempen B, de Sousa L (2019) Global mapping of soil salinity change. Remote Sens Environ 231:111260
Jesus JM, Danko AS, Fiúza A, Borges M-T (2015) Phytoremediation of salt-affected soils: a review of processes, applicability, and the impact of climate change. Environ Sci Pollut R 22:6511–6525
Jia B, Jia L, Mou X, Chen J, Li F, Ma Q, Li X (2022) Shrubification decreases soil organic carbon mineralization and its temperature sensitivity in alpine meadow soils. Soil Biol Biochem 168:108651
Jia Y, Liu Z, Zhou L, Liu X, Ma K, Feng X (2023) Soil organic carbon sourcing variance in the rhizosphere vs. non-rhizosphere of two mycorrhizal tree species. Soil Biol Biochem 176:108884
Jing C, Xu Z, Zou P, Tang Q, Li Y, You X, Zhang C (2019) Coastal halophytes alter properties and microbial community structure of the saline soils in the Yellow River Delta, China. Appl Soil Ecol 134:1–7
Joergensen RG (2018) Amino sugars as specific indices for fungal and bacterial residues in soil. Biol Fert Soils 54:559–568
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A (2010) Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol 187:843–858
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630
Khan KS, Mack R, Castillo X, Kaiser M, Joergensen RG (2016) Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 271:115–123
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Lai J, Zhou Y, Zhang J, Peres-Neto PR (2022) Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol Evol 13:782–788
Langer U, Böhme L, Böhme F (2004) Classification of soil microorganisms based on growth properties: a critical view of some commonly used terms. J Plant Nutr Soil Sc 167:267–269
Lavallee JM, Soong JL, Cotrufo MF (2019) Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Global Change Biol 26:261–273
Li L, Wilson CB, He H, Zhang X, Zhou F, Schaeffer SM (2019) Physical, biochemical, and microbial controls on amino sugar accumulation in soils under long-term cover cropping and no-tillage farming. Soil Biol Biochem 135:369–378
Liang C, Gutknecht JLM, Balser TC (2015) Microbial lipid and amino sugar responses to long-term simulated global environmental changes in a California annual grassland. Front Microbiol 6:1–11
Liang C, Jesus EdC, Duncan DS, Quensen JF, Jackson RD, Balser TC, Tiedje JM (2016) Switchgrass rhizospheres stimulate microbial biomass but deplete microbial necromass in agricultural soils of the upper Midwest, USA. Soil Biol Biochem 94:173–180
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biol 25:3578–3590
Lipson DA, Schmidt SK, Monson RK (1999) Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80:1623–1631
Liu Z, Liu X, Wu X, Bian R, Liu X, Zheng J, Zhang X, Cheng K, Li L, Pan G (2021) Long-term elevated CO2 and warming enhance microbial necromass carbon accumulation in a paddy soil. Biol Fert Soils 57:673–684
López-Mondéjar R, Brabcová V, Štursová M, Davidová A, Jansa J, Cajthaml T, Baldrian P (2018) Decomposer food web in a deciduous forest shows high share of generalist microorganisms and importance of microbial biomass recycling. ISME J 12:1768–1778
Malik AA, Martiny JBH, Brodie EL, Martiny AC, Treseder KK, Allison SD (2020) Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J 14:1–9
Marasco R, Fusi M, Ramond JB, Van Goethem MW, Seferji K, Maggs-Kölling G, Cowan DA, Daffonchio D (2022) The plant rhizosheath-root niche is an edaphic “mini-oasis” in hyperarid deserts with enhanced microbial competition. ISME Commun 2:47
Maslov MN, Maslova OA (2021) Nitrogen limitation of microbial activity in alpine tundra soils along an environmental gradient: Intra-seasonal variations and effect of rising temperature. Soil Biol Biochem 156:108234
Mavi MS, Sanderman J, Chittleborough DJ, Cox JW, Marschner P (2012) Sorption of dissolved organic matter in salt-affected soils: effect of salinity, sodicity and texture. Sci Total Environ 435–436:337–344
Moreno-Espíndola IP, Rivera-Becerril F, de Jesús F-G, De León-González F (2007) Role of root-hairs and hyphae in adhesion of sand particles. Soil Biol Biochem 39:2520–2526
Nie Y, Wang M, Zhang W, Ni Z, Hashidoko Y, Shen W (2018) Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci Total Environ 624:407–415
Olsen SR, Sommers LE (1982) Phosphorous. In: Page AL, Miller RH, Keeney DR (Eds) Methods of soil analysis, part 2, chemical and microbial properties. Agronomy Society of America, Agronomy Monograph 9, Madison, Wisconsin, 403–430
Park JH, Kalbitz K, Matzner E (2002) Resource control on the production of dissolved organic carbon and nitrogen in a deciduous forest floor. Soil Biol Biochem 34:813–822
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194
Pitman MG, Läuchli A (2002) Global impacts of salinity and agricultural ecosystem. In: Läuchli A, Lüttge U (eds) Salinity: environment-plants-molecules. Kluwer Academic, The Netherlands, pp 3–20
Pugnaire FI, Morillo JA, Peñuelas J, Reich PB, Bardgett RD, Gaxiola A, Wardle DA, van der Putten WH (2019) Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci adv 5:eaaz1834
Rathore AP, Chaudhary DR, Jha B (2017) Seasonal patterns of microbial community structure and enzyme activities in coastal saline soils of perennial halophytes. Land Degrad Dev 28:1779–1790
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Rewcastle KE, Moore JAM, Henning JA, Mayes MA, Patterson CM, Wang G, Metcalfe DB, Classen AT (2020) Investigating drivers of microbial activity and respiration in a forested bog. Pedosphere 30:135–145
Ruess RW, Hendrick RL, Burton AJ, Pregitzer KS, Sveinbjornsson B, Allen ME, Maurer GE (2003) Coupling fine root dynamics with ecosystem carbon cycling in black spruce forests of interior Alaska. Ecol Monogr 73:643–662
Sae-Tun O, Bodner G, Rosinger C, Zechmeister-Boltenstern S, Mentler A, Keiblinger K (2022) Fungal biomass and microbial necromass facilitate soil carbon sequestration and aggregate stability under different soil tillage intensities. Appl Soil Ecol 179:104599
Sardans J, Penuuelas J, Estiarte M, Prieto P (2008) Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Global Change Biol 14:2304–2316
Setia R, Marschner P, Baldock J, Chittleborough D, Smith P, Smith J (2011) Salinity effects on carbon mineralization in soils of varying texture. Soil Biol Biochem 43:1908–1916
Setia R, Rengasamy P, Marschner P (2013) Effect of exchangeable cation concentration on sorption and desorption of dissolved organic carbon in saline soils. Sci Total Environ 465:226–232
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 468:122
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53
Sokol NW, Kuebbing SE, Karlsen-Ayala E, Bradford MA (2019) Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol 221:233–246
Sokol NW, Slessarev E, Marschmann GL, Nicolas A, Blazewicz SJ, Brodie EL, Firestone MK, Foley MM, Hestrin R, Hungate BA, Koch BJ, Stone BW, Sullivan MB, Zablocki O, Pett-Ridge J (2022) Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat Rev Microbiol 20:415–430
Strickland MS, Rousk J (2010) Considering fungal:bacterial dominance in soils - methods, controls, and ecosystem implications. Soil Biol Biochem 42:1385–1395
Tian Q, Lu P, Zhai X, Zhang R, Zheng Y, Wang H, Nie B, Bai W, Niu S, Shi P, Yang Y, Li K, Yang D, Stevens C, Lambers H, Zhang W (2022) An integrated belowground trait-based understanding of nitrogen-driven plant diversity loss. Global Change Biol 28:3651–3664
Treonis AM, Ostle NJ, Stott AW, Primrose R, Grayston SJ, Ineson P (2004) Identification of groups of metabolically-active rhizosphere microorganisms by stable isotope probing of PLFAs. Soil Biol Biochem 36:533–537
Trivedi P, Batista BD, Bazany KE, Singh BK (2022) Plant–microbiome interactions under a changing world: responses, consequences and perspectives. New Phytol 234:1951–1959
Usyskin-Tonne A, Hadar Y, Yermiyahu U, Minz D (2021) Elevated CO2 and nitrate levels increase wheat root-associated bacterial abundance and impact rhizosphere microbial community composition and function. ISME J 15:1073–1084
Villarino SH, Pinto P, Jackson RB, Piñeiro G (2021) Plant rhizodeposition: a key factor for soil organic matter formation in stable fractions. Sci Adv 7:eabd3176
Wagg C, Schlaeppi K, Banerjee S, Kuramae EE, van der Heijden MGA (2019) Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat Commun 10:4841
Wang X, Yu J, Zhou D, Dong H, Li Y, Lin Q, Guan B, Wang Y (2012) Vegetative ecological characteristics of restored reed (Phragmites australis) wetlands in the Yellow River Delta, China. Environ Manage 49:325–333
Wang X, Sun R, Tian Y, Guo K, Sun H, Liu X, Chu H, Liu B (2020) Long-term phytoremediation of coastal saline soil reveals plant species-specific patterns of microbial community recruitment. mSystems 5:e00741-19
Wang B, Liang C, Yao H, Yang E, An S (2021a) The accumulation of microbial necromass carbon from litter to mineral soil and its contribution to soil organic carbon sequestration. Catena 207:105622
Wang C, Qu L, Yang L, Liu D, Morrissey E, Miao R, Liu Z, Wang Q, Fang Y, Bai E (2021b) Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Global Change Biol 27:2039–2048
Wang F, Che R, Deng Y, Wu Y, Tang L, Xu Z, Wang W, Liu H, Cui X (2021c) Air-drying and long time preservation of soil do not significantly impact microbial community composition and structure. Soil Biol Biochem 157:108238
Wang B, Huang Y, Li N, Yao H, Yang E, Soromotin AV, Kuzyakov Y, Cheptsov V, Yang Y, An S (2022) Initial soil formation by biocrusts: nitrogen demand and clay protection control microbial necromass accrual and recycling. Soil Biol Biochem 167:108607
Wen Z, White PJ, Shen J, Lambers H (2022) Linking root exudation to belowground economic traits for resource acquisition. New Phytol 233:1620–1635
West JR, Cates AM, Ruark MD, Deiss L, Whitman T, Rui Y (2020) Winter rye does not increase microbial necromass contributions to soil organic carbon in continuous corn silage in North Central US. Soil Biol Biochem 148:107899
Wichern J, Wichern F, Joergensen RG (2006) Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 137:100–108
Wu L, Wang Y, Zhang S, Wei W, Kuzyakov Y, Ding X (2021) Fertilization effects on microbial community composition and aggregate formation in saline-alkaline soil. Plant Soil 463:523–535
Xia Y, Chen X, Hu Y, Zheng S, Ning Z, Guggenberger G, He H, Wu J, Su Y (2019) Contrasting contribution of fungal and bacterial residues to organic carbon accumulation in paddy soils across eastern China. Biol Fert Soils 55:767–776
Xu X, Shi Z, Li D, Zhou X, Sherry RA, LuoY, (2015) Plant community structure regulates responses of prairie soil respiration to decadal experimental warming. Global Change Biol 21:3846–3853
Xu F, Liao H, Zhang Y, Yao M, Liu J, Sun L, Zhang X, Yang J, Wang K, Wang X, Ding Y, Liu C, Rensing C, Zhang J, Yeh K, Xu W (2022) Coordination of root auxin with the fungus Piriformospora indica and bacterium Bacillus cereus enhances rice rhizosheath formation under soil drying. ISME J 16:801–811
Yang Y, Xie H, Mao Z, Bao X, He H, Zhang X, Liang C (2022) Fungi determine increased soil organic carbon more than bacteria through their necromass inputs in conservation tillage croplands. Soil Biol Biochem 167:108587
Yin S, Liang G, Wang C, Zhou Z (2022) Asynchronous seasonal patterns of soil microorganisms and plants across biomes: a global synthesis. Soil Biol Biochem 175:108859
Zhang X, Amelung W (1996) Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol Biochem 28:1201–1206
Zhang H, Zai X, Wu X, Qin P, Zhang W (2014) An ecological technology of coastal saline soil amelioration. Ecol Eng 67:80–88
Zhang B, Chen S, Zhang J, He X, Liu W, Zhao Q, Zhao L, Tian C (2015) Depth-related responses of soil microbial communities to experimental warming in an alpine meadow on the Qinghai-Tibet Plateau. Eur J Soil Sci 66:496–504
Zhang W, Zhang X, Bai E, Cui Y, He H, Zhang X (2020) The strategy of microbial utilization of the deposited N in a temperate forest soil. Biol Fert Soils 56:359–367
Zhang X, Zhai P, Huang J (2022) Leaf carbon exchange of two dominant plant species impacted by water and nitrogen application in a semi-arid temperate steppe. Front Plant Sci 13:736009
Zhou Y, Staver AC (2019) Enhanced activity of soil nutrient-releasing enzymes after plant invasion: a meta-analysis. Ecology 100:e02830
Zhou J, Xue K, Xie J, Deng Y, Wu L, Cheng X, Fei S, Deng S, He Z, Van Nostrand JD, Luo Y (2012) Microbial mediation of carbon-cycle feedbacks to climate warming. Nat Clim Change 2:106–110
Zhu Y, Yu K, Wu Q, Cheng X, Li Z, Wang Z, Zhao M, Wilkes A, Bisselling T, Han G, Ren H (2023) Seasonal precipitation and soil microbial community influence plant growth response to warming and N addition in a desert steppe. Plant Soil 482:245–259
Funding
This study was supported by the National Key Research and Development Program (2022YFF1302804), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA23070202 and XDB40020000), National Natural Science Foundation of China (41977068, 41977105, and 42277349), and the Programs from Chinese Academy of Sciences (QYZDB-SSW-DQC039).
Author information
Authors and Affiliations
Contributions
Xiaorong Wei and Liping Qiu planned and designed the research; Tianhui Lu, Chunliang Chen, Zhenrui Cao, and Yueqing Yang performed experiments and conducted fieldwork; Tianhui Lu and Xiaorong Wei analyzed data and wrote the manuscript; Xiaorong Wei, Yaxian Hu, and Zekun Zhong revised the manuscript; Xiaomei Gou analyzed part of the data during revision; Benshuai Yan consulted literature and revised references during revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to missing reference.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, T., Chen, C., Qiu, L. et al. Halophyte functional groups influence seasonal variations in rhizosphere microbial necromass and enzyme activities in an inland saline ecosystem. Biol Fertil Soils 59, 989–1003 (2023). https://doi.org/10.1007/s00374-023-01768-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-023-01768-8