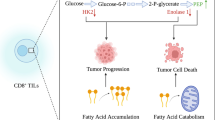
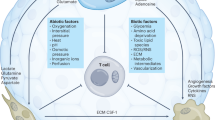
Abstract
Tumor cells must resist the host's immune system while maintaining growth under harsh conditions of acidity and hypoxia, which indicates that tumors are more robust than normal tissue. Immunotherapeutic agents have little effect on solid tumors, mostly because of the tumor density and the difficulty of penetrating deeply into the tissue to achieve the theoretical therapeutic effect. Various therapeutic strategies targeting the tumor microenvironment (TME) have been developed. Immunometabolic disorders play a dominant role in treatment resistance at both the TME and host levels. Understanding immunometabolic factors and their treatment potential may be a way forward for tumor immunotherapy. Here, we summarize the metabolism of substances that affect tumor progression, the crosstalk between the TME and immunosuppression, and some potential tumor-site targets. We also summarize the progress and challenges of tumor immunotherapy.




Similar content being viewed by others
Data availability
The data that support the fndings of this study are available from the corresponding author, upon reasonable request.
References
Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021. https://doi.org/10.1158/2159-8290.CD-20-1808.
Nia HT, Munn LL, Jain RK. Physical traits of cancer. Science. 2020. https://doi.org/10.1126/science.aaz0868.
Eelen G, Treps L, Li X, Carmeliet P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. 2020. https://doi.org/10.1161/CIRCRESAHA.120.316851.
Giannone G, Ghisoni E, Genta S, Scotto G, Tuninetti V, Turinetto M, et al. Immuno-metabolism and microenvironment in cancer: key players for immunotherapy. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21124414.
Kheshtchin N, Hadjati J. Targeting hypoxia and hypoxia-inducible factor-1 in the tumor microenvironment for optimal cancer immunotherapy. J Cell Physiol. 2022. https://doi.org/10.1002/jcp.30643.
Akkari L, Bowman RL, Tessier J, Klemm F, Handgraaf SM, de Groot M, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020. https://doi.org/10.1126/scitranslmed.aaw7843.
Seifert L, Werba G, Tiwari S, Giao Ly NN, Nguy S, Alothman S, et al. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology. 2016. https://doi.org/10.1053/j.gastro.2016.02.070.
Olson OC, Kim H, Quail DF, Foley EA, Joyce JA. Tumor-associated macrophages suppress the cytotoxic activity of antimitotic agents. Cell Rep. 2017. https://doi.org/10.1016/j.celrep.2017.03.038.
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011. https://doi.org/10.1158/2159-8274.CD-10-0028.
Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021. https://doi.org/10.1084/jem.20201606.
Chen AN, Luo Y, Yang YH, Fu JT, Geng XM, Shi JP, et al. Lactylation, a novel metabolic reprogramming code: current status and prospects. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.688910.
Hayes C, Donohoe CL, Davern M, Donlon NE. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 2021. https://doi.org/10.1016/j.canlet.2020.12.021.
Qian J, Rankin EB. Hypoxia-induced phenotypes that mediate tumor heterogeneity. Adv Exp Med Biol. 2019. https://doi.org/10.1007/978-3-030-12734-3_3.
Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021. https://doi.org/10.1186/s12943-020-01288-1.
Miranda-Galvis M, Teng Y. Targeting hypoxia-driven metabolic reprogramming to constrain tumor progression and metastasis. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155487.
Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020. https://doi.org/10.1186/s13046-020-01709-5.
Wei F, Wang D, Wei J, Tang N, Tang L, Xiong F, et al. Metabolic crosstalk in the tumor microenvironment regulates antitumor immunosuppression and immunotherapy resistance. Cell Mol Life Sci. 2021. https://doi.org/10.1007/s00018-020-03581-0.
You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2021. https://doi.org/10.1002/med.21771.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001. https://doi.org/10.1126/science.1059796.
Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008. https://doi.org/10.1038/cdd.2008.21.
Tafani M, Pucci B, Russo A, Schito L, Pellegrini L, Perrone GA, et al. Modulators of HIF1α and NFkB in cancer treatment: is it a rational approach for controlling malignant progression? Front Pharmacol. 2013. https://doi.org/10.3389/fphar.2013.00013.
Korbecki J, Kojder K, Barczak K, Simińska D, Gutowska I, Chlubek D, et al. Hypoxia alters the expression of CC chemokines and CC chemokine receptors in a tumor-a literature review. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21165647.
Weng CY, Kao CX, Chang TS, Huang YH. Immuno-metabolism: the role of cancer niche in immune checkpoint inhibitor resistance. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22031258.
DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016. https://doi.org/10.1126/sciadv.1600200.
Chen L, Huang L, Gu Y, Cang W, Sun P, Xiang Y. Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms231911943.
Ye L, Jiang Y, Zhang M. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev. 2022. https://doi.org/10.1016/j.cytogfr.2022.11.001.
Sun H, Zhu A, Zhou X, Wang F. Suppression of pyruvate dehydrogenase kinase-2 re-sensitizes paclitaxel-resistant human lung cancer cells to paclitaxel. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.16991.
Kolosenko I, Avnet S, Baldini N, Viklund J, De Milito A. Therapeutic implications of tumor interstitial acidification. Semin Cancer Biol. 2017. https://doi.org/10.1016/j.semcancer.2017.01.008.
Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012. https://doi.org/10.1016/j.freeradbiomed.2012.05.006.
Desbois M, Wang Y. Cancer-associated fibroblasts: key players in shaping the tumor immune microenvironment. Immunol Rev. 2021. https://doi.org/10.1111/imr.12982.
Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021. https://doi.org/10.1038/s41571-021-00546-5.
Xiang X, Niu YR, Wang ZH, Ye LL, Peng WB, Zhou Q. Cancer-associated fibroblasts: vital suppressors of the immune response in the tumor microenvironment. Cytokine Growth Factor Rev. 2022. https://doi.org/10.1016/j.cytogfr.2022.07.006.
Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021. https://doi.org/10.1152/physrev.00048.2019.
Galassi C, Musella M, Manduca N, Maccafeo E, Sistigu A. The immune privilege of cancer stem cells: a key to understanding tumor immune escape and therapy failure. Cells. 2021. https://doi.org/10.3390/cells10092361.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017. https://doi.org/10.1038/nm.4409.
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009. https://doi.org/10.1016/j.ccr.2009.03.018.
Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021. https://doi.org/10.1038/s41568-021-00366w.
Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022. https://doi.org/10.1186/s13046-022-02272-x.
Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics. 2021. https://doi.org/10.7150/thno.53749.
Jiang Z, Sun H, Yu J, Tian W, Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021. https://doi.org/10.1186/s13045-021-01197-w.
Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014. https://doi.org/10.1158/0008-5472.CAN-13-2563.
Ganesh K, Massagué J. TGF-β Inhibition and Immunotherapy: checkmate. Immunity. 2018. https://doi.org/10.1016/j.immuni.2018.03.037.
Kokubu Y, Tabu K, Muramatsu N, Wang W, Murota Y, Nobuhisa I, et al. Induction of protumoral CD11c(high) macrophages by glioma cancer stem cells through GM-CSF. Genes Cells. 2016. https://doi.org/10.1111/gtc.12333.
Yamashina T, Baghdadi M, Yoneda A, Kinoshita I, Suzu S, Dosaka-Akita H, et al. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res. 2014. https://doi.org/10.1158/0008-5472.CAN-13-2169.
Zhang Q, Cai DJ, Li B. Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Mol Med Rep. 2015. https://doi.org/10.3892/mmr.2015.3323.
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011. https://doi.org/10.1073/pnas.1106645108.
Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol Immunol. 2020. https://doi.org/10.1038/s41423-019-0344-8.
Utzschneider DT, Gabriel SS, Chisanga D, Gloury R, Gubser PM, Vasanthakumar A, et al. Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat Immunol. 2020. https://doi.org/10.1038/s41590-020-0760-z.
Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019. https://doi.org/10.1038/s41577-019-0180-1.
Kaiser M, Semeraro MD, Herrmann M, Absenger G, Gerger A, Renner W. Immune aging and immunotherapy in cancer. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22137016.
Yao C, Sun HW, Lacey NE, Ji Y, Moseman EA, Shih HY, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+ T cell persistence in chronic infection. Nat Immunol. 2019. https://doi.org/10.1038/s41590-019-0403-4.
Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019. https://doi.org/10.1038/s41586-019-1325-x.
Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022. https://doi.org/10.1016/j.ccell.2022.01.001.
Lopez Krol A, Nehring HP, Krause FF, Wempe A, Raifer H, Nist A, et al. Lactate induces metabolic and epigenetic reprogramming of pro-inflammatory Th17 cells. EMBO Rep. 2022. https://doi.org/10.15252/embr.202254685.
Wang H, Franco F, Ho PC. Metabolic regulation of tregs in cancer: opportunities for immunotherapy. Trends Cancer. 2017. https://doi.org/10.1016/j.trecan.2017.06.005.
Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020. https://doi.org/10.1186/s12943-020-01234-1.
Zhang C, Liu Y. Targeting NK cell checkpoint receptors or molecules for cancer immunotherapy. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.01295.
Bodac A, Meylan E. Neutrophil metabolism in the cancer context. Semin Immunol. 2021. https://doi.org/10.1016/j.smim.2021.101583.
Rakaee M, Busund LT, Paulsen EE, Richardsen E, Al-Saad S, Andersen S, et al. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.12360.
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0030806.
Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009. https://doi.org/10.1200/JCO.2008.18.9498.
Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coëlle C, Mouroux J, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012. https://doi.org/10.1002/cncr.26456.
Li S, Cong X, Gao H, Lan X, Li Z, Wang W, et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019. https://doi.org/10.1186/s13046-018-1003-0.
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-001946.
Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20030529.
Li J, Kumari T, Barazia A, Jha V, Jeong SY, Olson A, et al. Neutrophil DREAM promotes neutrophil recruitment in vascular inflammation. J Exp Med. 2022. https://doi.org/10.1084/jem.20211083.
Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016. https://doi.org/10.1053/j.gastro.2016.02.040.
Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2014. https://doi.org/10.1002/ijc.28622.
Leliefeld PH, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Front Immunol. 2015. https://doi.org/10.3389/fimmu.2015.00471.
Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021. https://doi.org/10.1038/s41571-021-00539-4.
Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.01749.
Kaltenmeier C, Yazdani HO, Morder K, Geller DA, Simmons RL, Tohme S. Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.785222.
Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021. https://doi.org/10.1016/j.ccell.2020.12.012.
Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-002305.
De Meo ML, Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. 2021. https://doi.org/10.1016/j.smim.2022.101595.
Stehr AM, Wang G, Demmler R, Stemmler MP, Krug J, Tripal P, et al. Neutrophil extracellular traps drive epithelial-mesenchymal transition of human colon cancer. J Pathol. 2022. https://doi.org/10.1002/path.5860.
Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2019. https://doi.org/10.1161/ATVBAHA.119.312463.
Wu L, Saxena S, Singh RK. Neutrophils in the tumor microenvironment. Adv Exp Med Biol. 2020. https://doi.org/10.1007/978-3-030-35723-8_1.
Sadiku P, Willson JA, Ryan EM, Sammut D, Coelho P, Watts ER, et al. Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis. Cell Metab. 2021. https://doi.org/10.1016/j.cmet.2020.11.016.
Wang H, Li J, Wang Y, Gong X, Xu X, Wang J, et al. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J Control Release. 2020. https://doi.org/10.1016/j.jconrel.2019.12.028.
Sen GA. Bio-inspired nanomedicine strategies for artificial blood components. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017. https://doi.org/10.1002/wnan.1464.
Liang X, Chen M, Bhattarai P, Hameed S, Dai Z. Perfluorocarbon@Porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. 2020. https://doi.org/10.1021/acsnano.0c05617.
Krafft MP. Alleviating tumor hypoxia with perfluorocarbon-based oxygen carriers. Alleviating Tumor Hypoxia Perfluorocarbon-Based Oxygen Carriers. 2020. https://doi.org/10.1016/j.coph.2020.08.010.
Jiao B, Liu S, Zhao H, Zhuang Y, Ma S, Lin C, et al. Hypoxia-responsive circRNAs: a novel but important participant in non-coding RNAs ushered toward tumor hypoxia. Cell Death Dis. 2022. https://doi.org/10.1038/s41419-022-05114-y.
Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q. Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine. 2021. https://doi.org/10.1016/j.ebiom.2021.103627.
Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. 2022. https://doi.org/10.1038/s41467-022-32521-8.
Elia I, Rowe JH, Johnson S, Joshi S, Notarangelo G, Kurmi K, et al. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8+ T cells. Cell Metab. 2022. https://doi.org/10.1016/j.cmet.2022.06.008.
Chen S, Zhou X, Yang X, Li W, Li S, Hu Z, et al. Dual blockade of lactate/GPR81 and PD-1/PD-L1 pathways enhances the anti-tumor effects of metformin. Biomolecules. 2021. https://doi.org/10.3390/biom11091373.
Zhu Y, Yang Z, Dong Z, Gong Y, Hao Y, Tian L, et al. CaCO3-assisted preparation of pH-responsive immune-modulating nanoparticles for augmented chemo-immunotherapy. Nanomicro Lett. 2020. https://doi.org/10.1007/s40820-020-00549-4.
Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020. https://doi.org/10.1016/j.ccell.2020.03.017.
Chen F, Wang Y, Gao J, et al. Nanobiomaterial-based vaccination immunotherapy of cancer. Biomaterials. 2021. https://doi.org/10.1016/j.biomaterials.2021.120709.
Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014. https://doi.org/10.1016/j.cmet.2014.05.004.
Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015. https://doi.org/10.1172/JCI76012.
Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017. https://doi.org/10.1016/j.cmet.2016.12.018.
Polański R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res. 2014. https://doi.org/10.1158/1078-0432.CCR-13-2270.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022. https://doi.org/10.1038/s41571-021-00588-9.
Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022. https://doi.org/10.1186/s12943-022-01569-x.
Guo H, Zhang W, Wang L, Shao Z, Huang X. Biomimetic cell membrane-coated glucose/oxygen-exhausting nanoreactor for remodeling tumor microenvironment in targeted hypoxic tumor therapy. Biomaterials. 2022. https://doi.org/10.1016/j.biomaterials.2022.121821.
Zhou S, Meng F, Du S, Qian H, Ding N, Sha H, et al. Bifunctional iRGD-anti-CD3 enhances antitumor potency of T cells by facilitating tumor infiltration and T-cell activation. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-001925.
Nahar UJ, Toth I, Skwarczynski M. Mannose in vaccine delivery. J Control Release. 2022. https://doi.org/10.1016/j.jconrel.2022.09.038.
Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022. https://doi.org/10.1186/s13045-022-01247-x.
Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017. https://doi.org/10.1038/nnano.2017.52.
Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020. https://doi.org/10.1038/s41586-020-2537-9.
Liu S, Jiang Q, Zhao X, Zhao R, Wang Y, Wang Y, et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater. 2021. https://doi.org/10.1038/s41563-020-0793-6.
Chen J, Ye Z, Huang C, Qiu M, Song D, Li Y, et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci U S A. 2022. https://doi.org/10.1073/pnas.2207841119.
Zhao X, Zhao R, Nie G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat Protoc. 2022. https://doi.org/10.1038/s41596-022-00713-7.
Oroojalian F, Beygi M, Baradaran B, Mokhtarzadeh A, Shahbazi MA. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021. https://doi.org/10.1002/smll.202006484.
Ukidve A, Cu K, Kumbhojkar N, Lahann J, Mitragotri S. Overcoming biological barriers to improve solid tumor immunotherapy. Drug Deliv Transl Res. 2021. https://doi.org/10.1007/s13346-021-00923-8.
Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013. https://doi.org/10.1016/j.bbrc.2013.09.037.
Assmann N, O’Brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM, et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol. 2017. https://doi.org/10.1038/ni.3838.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82060562), the Scientific and Technological Innovation Major Base of Guangxi (No. 2022-36-Z05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
This manuscript does not contain any human or animals related research, and does not involve any ethical experiments.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Nie, Y., Liu, X. et al. Tumor metabolic crosstalk and immunotherapy. Clin Transl Oncol 26, 797–807 (2024). https://doi.org/10.1007/s12094-023-03304-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03304-4