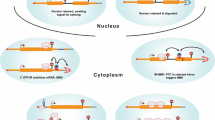
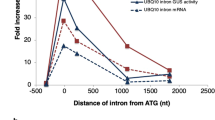
Abstract
The phenomenon of the positive influence of introns on the expression of a corresponding gene, which is called intron-mediated enhancement (IME), is characteristic of a wide variety of organisms, including nematodes, insects, mammals, fungi, and plants, and occurs due to an as-yet-undefined fundamental mechanism. IME introns have been used for a long time, in particular, in plant biotechnology. Understanding the mechanisms of this phenomenon allows predicting and easily generating stimulatory introns with the given properties and creating highly advantageous phenotypes. It will also give the greenlight to the use of IME in gene therapy and to improve the production of pharmaceutical proteins. In this review, we analysed previously proposed models of IME functioning mechanisms and identified factors that can directly or indirectly determine IME under different conditions and at different levels of gene expression, such as experimental methods of IME research, regulatory RNAs, sequence properties, intron position and orientation, factors at the levels of DNA, transcription, splicing, mRNA, translation, genes in which IME is detected, tissue specificity, repression and how some factors relate to each other by importance. Since there is no single mechanism of IME, and the effect may differ in different species, when modelling this process, only the cases of IME affecting the same level of expression should be compared with each other, taking into account the experimental conditions. Identifying the biological factors that may determine IME and the relationship between them will help in the future to create a corresponding data set suitable for machine learning and try to solve the mystery of the IME phenomenon using machine learning.


Similar content being viewed by others
REFERENCES
Agarwal, N.Yu. and Ansari, A., Enhancement of transcription by a splicing-competent intron is dependent on promoter directionality, PLoS Genet., 2016, vol. 12, no. 5, p. e1006047. https://doi.org/10.1371/journal.pgen.1006047
Akua, T. and Shaul, O., The Arabidopsis thaliana MHX gene includes an intronic element that boosts translation when localized in a 5' UTR intron, J. Exp. Bot., 2013, vol. 64, no. 14, pp. 4255–4270. https://doi.org/10.1093/jxb/ert235
Akua, T., Berezin, I., and Shaul, O., The leader intron of AtMHX can elicit, in the absence of splicing, low-level intron-mediated enhancement that depends on the internal intron sequence, BMC Plant Biol., 2010, vol. 10, p. 93. https://doi.org/10.1186/1471-2229-10-93
Al-Husini, N., Medler, S., and Ansari, A., Crosstalk of promoter and terminator during RNA polymerase II transcription cycle, Biochim. Biophys. Acta, Gene Regul. Mech., 2020, vol. 1863, no. 12, p. 194657. https://doi.org/10.1016/j.bbagrm.2020.194657
Alipanahi, B., Delong, A., Weirauch, M.T., et al., Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning, Nat. Biotechnol., 2015, vol. 33, no. 8, pp. 831–838. https://doi.org/10.1038/nbt.3300
Auslander, N., Gussow, A.B., and Koonin, E.V., Incorporating machine learning into established bioinformatics frameworks, Int. J. Mol. Sci., 2021, vol. 22, no. 6, p. 2903. https://doi.org/10.3390/ijms22062903
Baier, T., Jacobebbinghaus, N., Einhaus, A., et al., Introns mediate post-transcriptional enhancement of nuclear gene expression in the green microalga Chlamydomonas reinhardtii, PLoS Genet., 2020, vol. 16, no. 7, p. e1008944. https://doi.org/10.1371/journal.pgen.1008944
Barshai, M., Tripto, E., and Orenstein, Y., Identifying regulatory elements via deep learning, Annu. Rev. Biomed. Data Sci., 2020, vol. 3, pp. 315–338. https://doi.org/10.1146/annurev-biodatasci-022020-021940
Basso, M.F., Arraes, F.B.M., Grossi-de-Sa, M., et al., Insights into genetic and molecular elements for transgenic crop development, Front. Plant Sci., 2020, vol. 11, p. 509. https://doi.org/10.3389/fpls.2020.00509
Bhatti, G.K., Khullar, N., Sidhu, I.S., et al., Emerging role of non-coding RNA in health and disease, Metab. Brain Dis., 2021, vol. 36, no. 6, pp. 1119–1134. https://doi.org/10.1007/s11011-021-00739-y
Bieberstein, N.I., Carrillo Oesterreich, F., Straube, K., et al., First exon length controls active chromatin signatures and transcription, Cell Rep., 2012, vol. 2, no. 1, pp. 62–68. https://doi.org/10.1016/j.celrep.2012.05.019
Boehm, V. and Gehring, N.H., Exon junction complexes: supervising the gene expression assembly line, Trends Genet., 2016, vol. 32, no. 11, pp. 724–735. https://doi.org/10.1016/j.tig.2016.09.003
Bogard, B., Francastel, C., and Hubé, F., Multiple information carried by RNAs: total eclipse or a light at the end of the tunnel?, RNA Biol., 2020, vol. 17, no. 12, pp. 1707–1720. https://doi.org/10.1080/15476286.2020.1783868
Bourdon, V., Harvey, A., and Lonsdale, D.M., Introns and their positions affect the translational activity of mRNA in plant cells, EMBO Rep., 2001, vol. 2, no. 5, pp. 394–398. https://doi.org/10.1093/embo-reports/kve090
Bradnam, K.R. and Korf, I., Longer first introns are a general property of eukaryotic gene structure, PLoS One, 2008, vol. 3, no. 8, p. e3093. https://doi.org/10.1371/journal.pone.0003093
Callis, J., Fromm, M., and Walbot, V., Introns increase gene expression in cultured maize cells, Genes Dev., 1987, vol. 1, no. 10, pp. 1183–1200. https://doi.org/10.1101/gad.1.10.1183
Casas-Mollano, J.A., Lao, N.T., and Kavanagh, T.A., Intron-regulated expression of SUVH3, an Arabidopsis Su(var)3-9 homologue, J. Exp. Bot., 2006, vol. 57, no. 12, pp. 3301–3311. https://doi.org/10.1093/jxb/erl093
Chaubet-Gigot, N., Kapros, T., Flenet, M., et al., Tissue-dependent enhancement of transgene expression by introns of replacement histone H3 genes of Arabidopsis, Plant Mol Biol., 2001, vol. 45, no. 1, pp. 17–30. https://doi.org/10.1023/a:1006487023926
Chaudhary, S., Khokhar, W., Jabre, I., et al., Alternative splicing and protein diversity: plants versus animals, Front. Plant Sci., 2019, vol. 10, p. 708. https://doi.org/10.3389/fpls.2019.00708
Chicco, D., Ten quick tips for machine learning in computational biology, BioData Min., 2017, vol. 10, p. 35. https://doi.org/10.1186/s13040-017-0155-3
Chorev, M. and Carmel, L., The function of introns, Front. Genet., 2012, vol. 3, p. 55. https://doi.org/10.3389/fgene.2012.00055
Chung, B.Y., Simons, C., Firth, A.E., et al., Effect of 5′UTR introns on gene expression in Arabidopsis thaliana, BMC Genomics, 2006, vol. 7, p. 120. https://doi.org/10.1186/1471-2164-7-120
Clancy, M. and Hannah, L.C., Splicing of the maize Sh1 first intron is essential for enhancement of gene expression, and a T-rich motif increases expression without affecting splicing, Plant Physiol., 2002, vol. 130, no. 2, pp. 918–929. https://doi.org/10.1104/pp.008235
Clancy, M., Vasil, V., Hannah, C.L., et al., Maize Shrunken-1 intron and exon regions increase gene expression in maize protoplasts, Plant Sci., 2002, vol. 98, pp. 151–161. https://doi.org/10.1016/0168-9452(94)90005-1
Curi, G.C., Chan, R.L., and Gonzalez, D.H., The leader intron of Arabidopsis thaliana genes encoding cytochrome c oxidase subunit 5c promotes high-level expression by increasing transcript abundance and translation efficiency, J. Exp. Bot., 2005, vol. 56, pp. 419, pp. 2563–2571. https://doi.org/10.1093/jxb/eri250
Custódio, N. and Carmo-Fonseca, M., Co-transcriptional splicing and the CTD code, Crit. Rev. Biochem. Mol. B-iol., 2016, vol. 51, no. 5, pp. 395–411. https://doi.org/10.1080/10409238.2016.1230086
Damgaard, C.K., Kahns, S., Lykke-Andersen, S., et al., A 5′ splice site enhances the recruitment of basal transcription initiation factors in vivo, Mol. Cell., 2008, vol. 29, no. 2, pp. 271–278. https://doi.org/10.1016/j.molcel.2007.11.035
David-Assael, O., Berezin, I., Shoshani-Knaani, N., et al., AtMHX is an auxin and ABA-regulated transporter whose expression pattern suggests a role in metal homeostasis in tissues with photosynthetic potential, Funct. Plant Biol., 2006, vol. 33, no. 7, pp. 661–672. https://doi.org/10.1071/FP05295
Dean, C., Favreau, M., Bond-Nutter, D., et al., Sequences downstream of translation start regulate quantitative expression of two petunia rbcS genes, Plant Cell, 1989, vol. 1, no. 2, pp. 201–208. https://doi.org/10.1105/tpc.1.2.201
Depicker, A. and Montagu, M.V., Post-transcriptional gene silencing in plants, Curr. Opin. Cell Biol., 1997, vol. 9, no. 3, pp. 373–382. https://doi.org/10.1016/s0955-0674(97)80010-5
Donath, M., Mendel, R., Cerff, R., et al., Intron-dependent transient expression of the maize GapA1 gene, Plant Mol. Biol., 1995, vol. 28, no. 4, pp. 667–676. https://doi.org/10.1007/BF00021192
Dwyer, K., Agarwal, N., Gega, A., et al., Proximity to the promoter and terminator regions regulates the transcription enhancement potential of an intron, Front. Mol. Biosci., 2021a, vol. 8, p. 712639. https://doi.org/10.3389/fmolb.2021.712639
Dwyer, K., Agarwal, N., Pile, L., et al., Gene architecture facilitates intron-mediated enhancement of transcription, Front. Mol. Biosci., 2021b, vol. 8, p. 669004. https://doi.org/10.3389/fmolb.2021.669004
Eamens, A., Wang, M.B., Smith, N.A., et al., RNA silencing in plants: yesterday, today, and tomorrow, Plant Physiol., 2008, vol. 147, no. 2, pp. 456–468. https://doi.org/10.1104/pp.108.117275
Emami, S., Arumainayagam, D., Korf, I., et al., The effects of a stimulating intron on the expression of heterologous genes in Arabidopsis thaliana, Plant Biotechnol. J., 2013, vol. 11, no. 5, pp. 555–563. https://doi.org/10.1111/pbi.12043
Eraslan, G., Avsec, Ž., Gagneur, J., et al., Deep learning: new computational modelling techniques for genomics, Nat. Rev. Genet., 2019, vol. 20, no. 7, pp. 389–403. https://doi.org/10.1038/s41576-019-0122-6
Fagard, M. and Vaucheret, H., (Trans)gene silencing in plants: how many mechanisms?, Annu. Rev. Plant Physiol. Plant Mol. Biol., 2000, vol. 51, pp. 167–194. https://doi.org/10.1104/pp.108.117275
Fiume, E., Christou, P., Gianì, S., et al., Introns are key regulatory elements of rice tubulin expression, Planta, 2004, vol. 218, no. 5, pp. 693–703. https://doi.org/10.1007/s00425-003-1150-0
Furger, A., O’Sullivan, J.M., Binnie, A., et al., Promoter proximal splice sites enhance transcription, Genes Dev., 2002, vol. 16, no. 21, pp. 2792–2799. https://doi.org/10.1101/gad.983602
Gallegos, J.E. and Rose, A.B., The enduring mystery of intron-mediated enhancement, Plant Sci., 2015, vol. 237, pp. 8–15. https://doi.org/10.1016/j.plantsci.2015.04.017
Gallegos, J.E. and Rose, A.B., Intron DNA Sequences can be more important than the proximal promoter in determining the site of transcript initiation, Plant Cell., 2017, vol. 29, no. 4, pp. 843–853. https://doi.org/10.1105/tpc.17.00020
Gallegos, J.E. and Rose, A.B., An intron-derived motif strongly increases gene expression from transcribed sequences through a splicing independent mechanism in Arabidopsis thaliana, Sci. Rep., 2019, vol. 9, no. 1, p. 13777. https://doi.org/10.1038/s41598-019-50389-5
Gallois, J.L., Drouaud, J., Lécureuil, A., et al., Functional characterization of the plant ubiquitin regulatory X (UBX) domain-containing protein AtPUX7 in Arabidopsis thaliana, Gene, 2013, vol. 526, no. 2, pp. 299–308. https://doi.org/10.1016/j.gene.2013.05.056
Gianì, S., Altana, A., Campanoni, P., et al., In transgenic rice, α- and β-tubulin regulatory sequences control GUS amount and distribution through intron mediated enhancement and intron dependent spatial expression, Transgenic Res., 2009, vol. 18, no. 2, pp. 151–162. https://doi.org/10.1007/s11248-008-9202-7
Gidekel, M., Jimenez, B., and Herrera-Estrella, L., The first intron of the Arabidopsis thaliana gene coding for elongation factor 1β contains an enhancer-like element, Gene, 1996, vol. 170, no. 2, pp. 201–206. https://doi.org/10.1016/0378-1119(95)00837-3
Gromadzka, A.M., Steckelberg, A.L., Singh, K.K., et al., A short conserved motif in ALYREF directs cap- and EJC-dependent assembly of export complexes on spliced mRNAs, Nucleic Acids Res., 2016, vol. 44, no. 5, pp. 2348–2361. https://doi.org/10.1093/nar/gkw009
Hu, X., Fernie, A.R., and Yan, J., Deep learning in regulatory genomics: from identification to design, Curr. Opin. Biotechnol., 2023, vol. 79, p. 102887. https://doi.org/10.1016/j.copbio.2022.102887
Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W., GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants, EMBO J., 1987, vol. 6, no. 13, pp. 3901–3907. https://doi.org/10.1002/j.1460-2075.1987.tb02730.x
Jeon, J.S., Lee, S., Jung, K.H., et al., Tissue-preferential expression of a rice α-tubulin gene, OsTubA1, mediated by the first intron, Plant Physiol., 2000, vol. 123, no. 3, pp. 1005–1014. https://doi.org/10.1104/pp.123.3.1005
Jeong, Y.M., Mun, J.H., Lee, I., et al., Distinct roles of the first introns on the expression of Arabidopsis profilin gene family members, Plant Physiol., 2006, vol. 140, no. 1, pp. 196–209. https://doi.org/10.1104/pp.105.071316
Jeong, Y.M., Mun, J.H., Kim, H., et al., An upstream region in the first intron of petunia actin-depolymerizing factor 1 affects tissue-specific expression in transgenic Arabidopsis (Arabidopsis thaliana), Plant J., 2007, vol. 50, no. 2, pp. 230–239. https://doi.org/10.1111/j.1365-313X.2007.03053.x
Jo, B.S. and Choi, S.S., Introns: the functional benefits of introns in genomes, Genomics Inf., 2015, vol. 13, no. 4, pp. 112–118. https://doi.org/10.5808/GI.2015.13.4.112
Jo, S.S. and Choi, S.S., Enrichment of rare alleles within epigenetic chromatin marks in the first intron, Genomics Inf., 2019a, vol. 17, no. 1, p. e9. https://doi.org/10.5808/GI.2019.17.1.e9
Jo, S.S. and Choi, S.S., Analysis of the functional relevance of epigenetic chromatin marks in the first intron associated with specific gene expression patterns, Genome Bio-l. Evol., 2019b, vol. 11, no. 3, pp. 786–797. https://doi.org/10.1093/gbe/evz033
Karthikeyan, A.S., Ballachanda, D.N., and Raghothama, K.G. Promoter deletion analysis elucidates the role of cis elements and 5′UTR intron in spatiotemporal regulation of AtPht1;4 expression in Arabidopsis, Physiol. Plant., 2009, vol. 136, no. 1, pp. 10–18. https://doi.org/10.1111/j.1399-3054.2009.01207.x
Kertész, S., Kerényi, Z., Mérai, Z., et al., Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants, Nucleic Acids Res., 2006, vol. 34, no. 21, pp. 6147–6157. https://doi.org/10.1093/nar/gkl737
Kim, M.J., Kim, H., Shin, J.S., et al., Seed-specific expression of sesame microsomal oleic acid desaturase is controlled by combinatorial properties between negative cis-regulatory elements in the SeFAD2 promoter and enhancers in the 5'-UTR intron, Mol. Genet. Genomics, 2006, vol. 276, no. 4, pp. 351–368. https://doi.org/10.1007/s00438-006-0148-2
Kim, S., Kim, H., Fong, N., et al., Pre-mRNA splicing is a determinant of histone H3K36 methylation, Proc. Natl. Acad. Sci. U. S. A., 2011, vol. 108, no. 33, pp. 13564–13569. https://doi.org/10.1073/pnas.1109475108
Kooter, J.M., Matzke, M.A., and Meyer, P., Listening to the silent genes: transgene silencing, gene regulation and pathogen control, Trends Plant Sci., 1999, vol. 4, no. 9, pp. 340–347. https://doi.org/10.1016/s1360-1385(99)01467-3
Korf, I.F. and Rose, A.B., Applying word-based algorithms: The IMEter, Methods Mol. Biol., 2009, vol. 553, pp. 287–301. https://doi.org/10.1007/978-1-60327-563-7_14
Laxa, M., Intron-mediated enhancement: a tool for heterologous gene expression in plants?, Front. Plant Sci., 2017, vol. 7, p. 1977. https://doi.org/10.3389/fpls.2016.01977
Laxa, M., Müller, K., Lange, N., et al., The 5'UTR intron of Arabidopsis GGT1 aminotransferase enhances promoter activity by recruiting RNA polymerase II, Plant Physiol., 2016, vol. 172, no. 1, pp. 313–327. https://doi.org/10.1104/pp.16.00881
Le Hir, H., Gatfield, D., Izaurralde, E., et al., The exonexon junction complex provides a binding platform for factors involved in mRNA export and nonsense mediated mRNA decay, EMBO J., 2001, vol. 20, no. 17, pp. 4987–4997. https://doi.org/10.1093/emboj/20.17.4987
Le Hir, H., Nott, A., and Moore, M.J., How introns influence and enhance eukaryotic gene expression, Trends Biochem. Sci., 2003, vol. 28, no. 4, pp. 215–220. https://doi.org/10.1016/S0968-0004(03)00052-5
Le Hir, H., Saulière, J., and Wang, Z., The exon junction complex as a node of post-transcriptional networks, Nat. Rev. Mol. Cell Biol., 2016, vol. 17, no. 1, pp. 41–54. https://doi.org/10.1038/nrm.2015.7
Liao, L., Ning, G., Liu, C., et al., The intron from the 5'-UTR of the FBP11 gene in petunia displays promoter- and enhancer-like functions, Sci Hortic., 2013, vol. 154, pp. 96–101. https://doi.org/10.1016/j.scienta.2013.02.009
Lim, C.S.T., Wardell, S.J., Kleffmann, T., et al., The exon-intron gene structure upstream of the initiation codon predicts translation efficiency, Nucleic Acids Res., 2018, vol. 46, no. 9, pp. 4575–4591. https://doi.org/10.1093/nar/gky282
Lu, S. and Cullen, B.R., Analysis of the stimulatory effect of splicing on mRNA production and utilization in mammalian cells, RNA, 2003, vol. 9, no. 5, pp. 618–630. https://doi.org/10.1261/rna.5260303
Lu, J., Sivamani, E., Li, X., et al., Activity of the 5' regulatory regions of the rice polyubiquitin rubi3 gene in transgenic rice plants as analyzed by both GUS and GFP reporter genes, Plant Cell Rep., 2008, vol. 27, no. 10, pp. 1587–1600. https://doi.org/10.1007/s00299-008-0577-y
Luehrsen, K.R. and Walbot, V., Intron enhancement of gene expression and the splicing efficiency of introns in maize cells, Mol. Gen. Genet., 1991, vol. 225, no. 1, pp. 81–93. https://doi.org/10.1007/BF00282645
Maas, C., Laufs, J., Grant, S., et al., The combination of a novel stimulatory element in the first exon of the maize Shrunken-1 gene with the following intron 1 enhances reporter gene expression up to 1000-fold, Plant Mol. Bi-ol., 1991, vol. 16, no. 2, pp. 199–207. https://doi.org/10.1007/BF00020552
Mascarenhas, D., Mettler, I.J., Pierce, D.A., et al., Intron-mediated enhancement of heterologous gene expression in maize, Plant Mol. Biol., 1990, vol. 15, no. 6, pp. 913–920. https://doi.org/10.1007/BF00039430
McElroy, D., Zhang, W., Cao, J., et al., Isolation of an efficient actin promoter for use in rice transformation, Plant Cell, 1990, vol. 2 no. 2, pp. 163–171. https://doi.org/10.1105/tpc.2.2.163
Meagher, R.B., McKinney, E.C., and Vitale, A.V., The evolution of new structures: clues from plant cytoskeletal genes, Trends Genet., 1999, vol. 15, no. 7, pp. 278–284. https://doi.org/10.1016/s0168-9525(99)01759-x
Moabbi, A.M., Agarwal, N., El Kaderi, B., et al., Role for gene looping in intron-mediated enhancement of transcription, Proc. Natl. Acad. Sci. U. S. A., 2012, vol. 109, no. 22, pp. 8505–8510. https://doi.org/10.1073/pnas.1112400109
Morello, L. and Breviario, D., Plant spliceosomal introns: not only cut and paste, Curr. Genomics, 2008, vol. 9, no. 4, pp. 227–238. https://doi.org/10.2174/138920208784533629
Morello, L., Bardini, M., Sala, F., et al., A long leader intron of the Ostub16 rice β-tubulin gene is required for high-level gene expression and can autonomously promote transcription both in vivo and in vitro, Plant J., 2002, vol. 29, no. 1, pp. 33–44. https://doi.org/10.1046/j.0960-7412.2001.01192.x
Morello, L., Bardini, M., Cricrì, M., et al., Functional analysis of DNA sequences controlling the expression of the rice OsCDPK2 gene, Planta, 2006, vol. 223, no. 3, pp. 479–491. https://doi.org/10.1007/s00425-005-0105-z
Morello, L., Gianì, S., Troina, F., et al., Testing the IMEter on rice introns and other aspects of intron-mediated enhancement of gene expression, J. Exp. Bot., 2011, vol. 62, no. 2, pp. 533–544. https://doi.org/10.1093/jxb/erq273
Morita, S., Tsukamoto, S., Sakamoto, A., et al., Differences in intron-mediated enhancement of gene expression by the first intron of cytosolic superoxide dismutase gene from rice in monocot and dicot plants, Plant Biotech. J., 2012, vol. 29, pp. 115–119. https://doi.org/10.5511/plantbiotechnology.11.1207a
Mun, J.H., Lee, S.Y., Yu, H.J., et al., Petunia actin-depolymerizing factor is mainly accumulated in vascular tissue and its gene expression is enhanced by the first intron, Gene, 2002, vol. 292, nos. 1–2, pp. 233–243. https://doi.org/10.1016/s0378-1119(02)00646-7
Nesic, D., Cheng, J., and Maquat, L.E., Sequences within the last intron function in RNA 3'-end formation in cultured cells, Mol. Cell Biol., 1993, vol. 13, no. 6, pp. 3359–3369. https://doi.org/10.1128/mcb.13.6.3359-3369.1993
Niu, D.K. and Yang, Y.F., Why eukaryotic cells use introns to enhance gene expression: Splicing reduces transcription-associated mutagenesis by inhibiting topoisomerase I cutting activity, Biol. Direct, 2011, vol. 6, p. 24. https://doi.org/10.1186/1745-6150-6-24
O’Sullivan, J.M., Tan-Wong, S.M., Morillon, A., et al., Gene loops juxtapose promoters and terminators in yeast, Nat. Genet., 2004, vol. 36, no. 9, pp. 1014–1018. https://doi.org/10.1038/ng1411
Park, S.G., Hannenhalli, S., and Choi, S.S., Conservation in first introns is positively associated with the number of exons within genes and the presence of regulatory epigenetic signals, BMC Genomics, 2014, vol. 15, no. 1, p. 526. https://doi.org/10.1186/1471-2164-15-526
Parra, G., Bradnam, K., Rose, A.B., et al., Comparative and functional analysis of intron-mediated enhancement signals reveals conserved features among plants, Nucleic Acids Res., 2011, vol. 39, no. 13, pp. 5328–5337. https://doi.org/10.1093/nar/gkr043
Plesse, B., Criqui, M.C., Durr, A., et al., Effects of the polyubiquitin gene Ubi.U4 leader intron and first ubiquitin monomer on reporter gene expression in Nicotiana tabacum, Plant Mol. Biol., 2001, vol. 45, no. 6, pp. 655–667. https://doi.org/10.1023/a:1010671405594
Proudfoot, N.J., Furger, A., and Dye, M.J., Integrating mRNA processing with transcription, Cell, 2002, vol. 108, no. 4, pp. 501–512. https://doi.org/10.1016/s0092-8674(02)00617-7
Que, Q., Chilton, M.D., de Fontes, C.M., et al., Trait stacking in transgenic crops: challenges and opportunities, GM Crops, 2010, vol. 1, no. 4, pp. 220–229. https://doi.org/10.4161/gmcr.1.4.13439
Rethmeier, N., Seurinck, J., Van Montagu, M., et al., Intron-mediated enhancement of transgene expression in maize is a nuclear, gene-dependent process, Plant J., 1997, vol. 12, no. 4, pp. 895–899. https://doi.org/10.1046/j.1365-313x.1997.12040895.x
Rogozin, I.B., Carmel, L., Csuros, M., et al., Origin and evolution of spliceosomal introns, Biol. Direct, 2012, vol. 7, p. 11. https://doi.org/10.1186/1745-6150-7-11
Rose, A.B., Requirements for intron-mediated enhancement of gene expression in Arabidopsis, RNA, 2002, vol. 8, no. 11, pp. 1444–1453. https://doi.org/10.1017/s1355838202020551
Rose, A.B., The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis, Plant J., 2004, vol. 40, no. 5, pp. 744–751. https://doi.org/10.1111/j.1365-313X.2004.02247.x
Rose, A.B., Intron-mediated regulation of gene expression, Curr. Top. Microbiol. Immunol., 2008, vol. 326, pp. 277–290. https://doi.org/10.1007/978-3-540-76776-3_15
Rose, A.B., Introns as gene regulators: a brick on the accelerator, Front. Genet., 2019, vol. 9, p. 672. https://doi.org/10.3389/fgene.2018.00672
Rose, A.B. and Beliakoff, J.A., Intron-mediated enhancement of gene expression independent of unique intron sequences and splicing, Plant Physiol., 2000, vol. 122, no. 2, pp. 535–542. https://doi.org/10.1104/pp.122.2.535
Rose, A.B. and Last, R.L., Introns act post-transcriptionally to increase expression of the Arabidopsis thaliana tryptophan pathway gene PAT1, Plant J., 1997, vol. 11, no. 3, pp. 455–464. https://doi.org/10.1046/j.1365-313x.1997.11030455.x
Rose, A.B., Elfersi, T., Parra, G., et al., Promoterproximal introns in Arabidopsis thaliana are enriched in dispersed signals that elevate gene expression, Plant Cell, 2008, vol. 20, no. 3, pp. 543–551. https://doi.org/10.1105/tpc.107.057190
Rose, A.B., Emami, S., Bradnam, K., et al., Evidence for a DNA-based mechanism of intron-mediated enhancement, Front. Plant Sci., 2011, vol. 2, p. 98. https://doi.org/10.3389/fpls.2011.00098
Rose, A.B., Carter, A., Korf, I., et al., Intron sequences that stimulate gene expression in Arabidopsis, Plant Mol. Biol., 2016, vol. 92, no. 3, pp. 337–346. https://doi.org/10.1007/s11103-016-0516-1
Rowlands, C.F., Baralle, D., and Ellingford, J.M., Machine learning approaches for the prioritization of genomic variants impacting pre-mRNA splicing, Cells, 2019, vol. 8, no. 12, p. 1513. https://doi.org/10.3390/cells8121513
Salgueiro, S., Pignocchi, C., and Parry, M.A., Intronmediated gusA expression in tritordeum and wheat resulting from particle bombardment, Plant Mol. Biol., 2000, vol. 42, no. 4, pp. 615–622. https://doi.org/10.1023/a:1006331831858
Samadder, P., Sivamani, E., Lu, J., et al., Transcriptional and post-transcriptional enhancement of gene expression by the 5' UTR intron of rice rubi3 gene in transgenic rice cells, Mol. Genet. Genomics, 2008, vol. 279, no. 4, pp. 429–439. https://doi.org/10.1007/s00438-008-0323-8
Shaul, O., Unique aspects of plant nonsense-mediated mRNA decay, Trends Plant Sci., 2015, vol. 20, no. 11, pp. 767–779. https://doi.org/10.1016/j.tplants.2015.08.011
Shaul, O., How introns enhance gene expression, Int. J. Biochem. Cell Biol., 2017, vol. 91, pp. 145–155. https://doi.org/10.1016/j.biocel.2017.06.016
Shen, X., Jiang, C., Wen, Y., et al., A Brief review on deep learning applications in genomic studies, Front. Syst. Biol., 2022, vol. 2, p. 877717. https://doi.org/10.3389/fsysb.2022.877717
Silva, J.C.F., Teixeira, R.M., Silva, F.F., et al., Machine learning approaches and their current application in plant molecular biology: A systematic review, Plant Sci., 2019, vol. 284, pp. 37–47. https://doi.org/10.1016/j.plantsci.2019.03.020
Simna, S.P. and Han, Z., Prospects of non-coding elements in genomic DNA based gene therapy, Curr. Gene Ther., 2022, vol. 22, no. 2, pp. 89–103. https://doi.org/10.2174/1566523221666210419090357
Sinibaldi, R.M. and Mettler, I.J., Intron splicing and intron-mediated enhanced expression in monocots, Prog. Nucleic Acid Res. Mol. Biol., 1992, vol. 42, pp. 229–257. https://doi.org/10.1016/s0079-6603(08)60577-2
Snowden, K.C., Buchhholz, W.G., and Hall, T.C., Intron position affects expression from the tpi promoter in rice, Plant Mol. Biol., 1996, vol. 31, no. 3, pp. 689–692. https://doi.org/10.1007/BF00042241
Spies, N., Nielsen, C.B., Padgett, R.A., et al., Biased chromatin signatures around polyadenylation sites and exons, Mol. Cell, 2009, vol. 36, no. 2, pp. 245–254. https://doi.org/10.1016/j.molcel.2009.10.008
Tanaka, A., Mita, S., Ohta, S., et al., Enhancement of foreign gene expression by a dicot intron in rice but not in tobacco is correlated with an increased level of mRNA and an efficient splicing of the intron, Nucleic Acids Res., 1990, vol. 18, no. 23, pp. 6767–6770. https://doi.org/10.1093/nar/18.23.6767
To, J.P.C., Davis, I.W., Marengo, M.S., et al., Expression elements derived from plant sequences provide effective gene expression regulation and new opportunities for plant biotechnology traits, Front. Plant Sci., 2021, vol. 12, p. 712179. https://doi.org/10.3389/fpls.2021.712179
Travella, S., Ross, S.M., Harden, J., et al., A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques, Plant Cell Rep., 2005, vol. 23, no. 12, pp. 780–789. https://doi.org/10.1007/s00299-004-0892-x
Valencia, P., Dias, A.P., and Reed, R., Splicing promotes rapid and efficient mRNA export in mammalian cells, Proc. Natl. Acad. Sci. U. S. A., 2008, vol. 105, no. 9, pp. 3386–3391. https://doi.org/10.1073/pnas.0800250105
Vasil, V., Clancy, M., Ferl, R.J., et al., Increased gene expression by the first intron of maize shrunken-1 locus in grass species, Plant Physiol., 1989, vol. 91, no. 4, pp. 1575–1579. https://doi.org/10.1104/pp.91.4.1575
Vaucheret, H., Béclin, C., Elmayan, T., et al., Transgene-induced gene silencing in plants, Plant J., 1998, vol. 16, no. 6, pp. 651–659. https://doi.org/10.1046/j.1365-313x.1998.00337.x
Vitale, A., Wu, R.J., Cheng, Z., et al., Multiple conserved 5' elements are required for high-level pollen expression of the Arabidopsis reproductive actin ACT1, Plant Mol. Biol., 2003, vol. 52, no. 6, pp. 1135–1151. https://doi.org/10.1023/b:plan.0000004309.06973.16
Weise, A., Rodriguez-Franco, M., Timm, B., et al., Use of Physcomitrella patens actin 5′ regions for high transgene expression: importance of 5' introns, Appl. Microbiol. Biotechnol., 2006, vol. 70, no. 3, pp. 337–345. https://doi.org/10.1007/s00253-005-0087-6
Xiao, G., Zhang, Z.Q., Yin, C.F., et al., Characterization of the promoter and 5'-UTR intron of oleic acid desaturase (FAD2) gene in Brassica napus, Gene, 2014, vol. 545, no. 1, pp. 45–55. https://doi.org/10.1016/j.gene.2014.05.008
Zalabák, D. and Ikeda, Y., First come, first served: sui generis features of the first intron, Plants, 2020, vol. 9, no. 7, p. 911. https://doi.org/10.3390/plants9070911
Zhang, Y., Yan, J., Chen, S., et al., A Review on the application of deep learning in bioinformatics, Curr. Bioinf., 2020, vol. 15, no. 8, pp. 898–911. https://doi.org/10.2174/1574893615999200711165743
Zou, J., Huss, M., Abid, A., et al., A primer on deep learning in genomics, Nat. Genet., 2019, vol. 51, no. 1, pp. 12–18. https://doi.org/10.1038/s41588-018-0295-5
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
About this article
Cite this article
Pydiura, M.O., Blume, Y.B. Mechanisms of Intron-Mediated Enhancement of Expression: Welcome to the Hotel California. Cytol. Genet. 57, 413–431 (2023). https://doi.org/10.3103/S0095452723050055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452723050055