Article contents
Minerals with a palmierite-type structure. Part II. Nomenclature and classification of the palmierite supergroup.
Published online by Cambridge University Press: 24 July 2023
Abstract
The palmierite supergroup, approved by the IMA-CNMNC, includes five mineral species characterised by the general crystal-chemical formula XIIM1XM22(IVTO4)2 (Z = 3). On the basis of the crystal-chemical arguments and heterovalent isomorphic substitution scheme M++T6+ ↔ M2++T5+, the palmierite supergroup can be formally divided into two groups: the palmierite group M12+M22+(T6+O4)2, and the tuite group M12+M222+(T5+O4)2. Currently, the palmierite group includes palmierite K2Pb(SO4)2, and kalistrontite K2Sr(SO4)2, whereas the tuite group combines tuite Ca3(PO4)2, mazorite Ba3(PO4)2, and gurimite Ba3(VO4)2. The isostructural supergroup members crystallise in space group R$\bar{3}$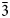 m (no. 166). The palmierite-type crystal structure is characterised by a sheet arrangement composed of layers formed by M1O12 and M2O10 polyhedra separated by TO4 tetrahedra perpendicular to the c axis. The abundance of distinct ions, which may be hosted at the M and T sites (M = K, Na, Ca, Sr, Ba, Sr, Pb, Rb, Zn, Tl, Cs, Bi, NH4 and REE; T = Si, P, V, As, S, Se, Mo, Cr and W) implies many possible combinations, resulting in potentially new mineral species. Minerals belonging to the palmierite supergroup are relatively rare and usually form under specific conditions, and their synthetic counterparts play a significant role in various industrial applications.
m (no. 166). The palmierite-type crystal structure is characterised by a sheet arrangement composed of layers formed by M1O12 and M2O10 polyhedra separated by TO4 tetrahedra perpendicular to the c axis. The abundance of distinct ions, which may be hosted at the M and T sites (M = K, Na, Ca, Sr, Ba, Sr, Pb, Rb, Zn, Tl, Cs, Bi, NH4 and REE; T = Si, P, V, As, S, Se, Mo, Cr and W) implies many possible combinations, resulting in potentially new mineral species. Minerals belonging to the palmierite supergroup are relatively rare and usually form under specific conditions, and their synthetic counterparts play a significant role in various industrial applications.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press on behalf of The Mineralogical Society of the United Kingdom and Ireland
Footnotes
Associate Editor: Oleg I Siidra
References
- 1
- Cited by




