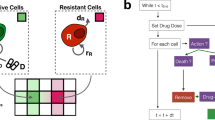
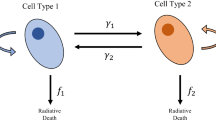
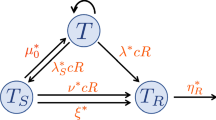
Abstract
In cancer treatment, adaptive therapy holds promise for delaying the onset of recurrence through regulating the competition between drug-sensitive and drug-resistant cells. Adaptive therapy has been studied in well-mixed models assuming free mixing of all cells and spatial models considering the interactions of single cells with their immediate adjacent cells. Both models do not reflect the spatial structure in glandular tumours where intra-gland cellular interaction is high, while inter-gland interaction is limited. Here, we use mathematical modelling to study the effects of adaptive therapy on glandular tumours that expand using either glandular fission or invasive growth. A two-dimensional, lattice-based model of sites containing sensitive and resistant cells within individual glands is developed to study the evolution of glandular tumour cells under continuous and adaptive therapies. We found that although both growth models benefit from adaptive therapy’s ability to prevent recurrence, invasive growth benefits more from it than fission growth. This difference is due to the migration of daughter cells into neighboring glands that is absent in fission but present in invasive growth. The migration resulted in greater mixing of cells, enhancing competition induced by adaptive therapy. By varying the initial spatial spread and location of the resistant cells within the tumour, we found that modifying the conditions within the resistant cells containing glands affect both fission and invasive growth. However, modifying the conditions surrounding these glands affect invasive growth only. Our work reveals the interplay between growth mechanism and tumour topology in modulating the effectiveness of cancer therapy.
Graphical Abstract








Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/CAAC.21654
Holohan C, Van Schaeybroeck S, Longley DB et al (2013) Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13(10):714–726. https://doi.org/10.1038/nrc3599
Marusyk A, Janiszewska M, Polyak K (2020) Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell 37:471. https://doi.org/10.1016/J.CCELL.2020.03.007
Vineis P, Berwick M (2006) The population dynamics of cancer: a Darwinian perspective. Int J Epidemiol 35:1151–1159. https://doi.org/10.1093/IJE/DYL185
Juliano SA (2007) Population dynamics. J Am Mosq Control Assoc 23:265. https://doi.org/10.2987/8756-971x(2007)23[265:pd]2.0.co;2
Kam Y, Das T, Tian H et al (2015) Sweat but no gain: inhibiting proliferation of multidrug resistant cancer cells with “ersatzdroges.” Int J Cancer 136:E188–E196. https://doi.org/10.1002/IJC.29158
Herrmann NC, Stroud JT, Losos JB (2021) The evolution of ‘ecological release’ into the 21st century. Trends Ecol Evol 36:206–215. https://doi.org/10.1016/j.tree.2020.10.019
Melander AL (1914) Can insects become resistant to sprays? J Econ Entomol 7:167–173. https://doi.org/10.1093/JEE/7.2.167
Abel zur Wiesch P, Kouyos R, Abel S, et al (2014) Cycling empirical antibiotic therapy in hospitals: meta-analysis and models. PLoS Pathog 10:e1004225. https://doi.org/10.1371/JOURNAL.PPAT.1004225
Gatenby RA, Silva AS, Gillies RJ et al (2009) Adaptive therapy. Cancer Res 69:4894–4903. https://doi.org/10.1158/0008-5472.CAN-08-3658
Bacevic K, Noble R, Soffar A et al (2017) Spatial competition constrains resistance to targeted cancer therapy. Nat Commun 8:1–15. https://doi.org/10.1038/s41467-017-01516-1
Enriquez-Navas PM, Kam Y, Das T et al (2016) Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med 8(327):327ra24. https://doi.org/10.1126/SCITRANSLMED.AAD7842
Smalley I, Kim E, Li J et al (2019) Leveraging transcriptional dynamics to improve BRAF inhibitor responses in melanoma. EBioMedicine 48:178–190. https://doi.org/10.1016/j.ebiom.2019.09.023
Acar A, Nichol D, Fernandez-Mateos J et al (2020) Exploiting evolutionary steering to induce collateral drug sensitivity in cancer. Nat Commun 11:1–14. https://doi.org/10.1038/s41467-020-15596-z
Viossat Y, Noble R (2021) A theoretical analysis of tumour containment. Nat Ecol Evol 5:826–835. https://doi.org/10.1038/s41559-021-01428-w
Martin RB, Fisher ME, Minchin RF et al (1992) Optimal control of tumor size used to maximize survival time when cells are resistant to chemotherapy. Math Biosci 110:201–219. https://doi.org/10.1016/0025-5564(92)90038-X
Zhang J, Cunningham JJ, Brown JS et al (2017) Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun 8:1–9. https://doi.org/10.1038/s41467-017-01968-5
West J, Ma Y, Newton PK (2018) Capitalizing on competition: an evolutionary model of competitive release in metastatic castration resistant prostate cancer treatment. J Theor Biol 455:249–260. https://doi.org/10.1016/J.JTBI.2018.07.028
Gallaher JA, Enriquez-Navas PM, Luddy KA et al (2018) Spatial heterogeneity and evolutionary dynamics modulate time to recurrence in continuous and adaptive cancer therapies. Cancer Res 78:2127–2139. https://doi.org/10.1158/0008-5472.CAN-17-2649
Italia M, Dercole F, Lucchetti R (2022) Optimal chemotherapy counteracts cancer adaptive resistance in a cell-based, spatially-extended, evolutionary model. Phys Biol. 19(2): 026004 https://doi.org/10.1088/1478-3975/AC509C
Akhmetzhanov AR, Kim JW, Sullivan R et al (2019) Modelling bistable tumour population dynamics to design effective treatment strategies. J Theor Biol 474:88–102. https://doi.org/10.1016/J.JTBI.2019.05.005
Weiner BG, Posfai A, Wingreen NS (2019) Spatial ecology of territorial populations. Proc Natl Acad Sci USA 116:17874–17879. https://doi.org/10.1073/PNAS.1911570116/SUPPL_FILE/PNAS.1911570116.SAPP.PDF
Apps CD, McLellan BN, Woods JG (2006) Landscape partitioning and spatial inferences of competition between black and grizzly bears. Ecography 29:561–572. https://doi.org/10.1111/J.0906-7590.2006.04564.X
Lynch MD, Lynch CNS, Craythorne E et al (2017) Spatial constraints govern competition of mutant clones in human epidermis. Nat Commun 8:1–11. https://doi.org/10.1038/s41467-017-00993-8
Lloyd DP, Allen RJ (2015) Competition for space during bacterial colonization of a surface. J R Soc Interface. https://doi.org/10.1098/RSIF.2015.0608
Noble R, Burri D, Le Sueur C et al (2021) Spatial structure governs the mode of tumour evolution. Nat Ecol Evol 6:207–217. https://doi.org/10.1038/s41559-021-01615-9
Waclaw B, Bozic I, Pittman ME et al (2015) A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature 525(7568):261–264. https://doi.org/10.1038/nature14971
Strobl MAR, Gallaher J, West J et al (2022) Spatial structure impacts adaptive therapy by shaping intra-tumoral competition. Commun Med 2:1–18. https://doi.org/10.1038/s43856-022-00110-x
Makki J (2015) Diversity of breast carcinoma: histological subtypes and clinical relevance. Clin Med Insights Pathol 8:23. https://doi.org/10.4137/CPATH.S31563
Fleming M, Ravula S, Tatishchev SF et al (2012) Colorectal carcinoma: pathologic aspects. J Gastrointest Oncol 3:153. https://doi.org/10.3978/J.ISSN.2078-6891.2012.030
Dela Cruz CS, Tanoue LT, Matthay RA (2011) Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32:605–644. https://doi.org/10.1016/J.CCM.2011.09.001
Ryser MD, Min BH, Siegmund KD et al (2018) Spatial mutation patterns as markers of early colorectal tumor cell mobility. Proc Natl Acad Sci USA 115:5774–5779. https://doi.org/10.1073/PNAS.1716552115/-/DCSUPPLEMENTAL
Sun R, Hu Z, Sottoriva A et al (2017) Between-region genetic divergence reflects the mode and tempo of tumor evolution. Nat Genet 49(7):1015–1024. https://doi.org/10.1038/ng.3891
Pandya P, Orgaz JL, Sanz-Moreno V (2017) Modes of invasion during tumour dissemination. Mol Oncol 11:5–27. https://doi.org/10.1002/1878-0261.12019
Lugli A, Zlobec I, Berger MD et al (2020) Tumour budding in solid cancers. Nat Rev Clin Oncol 18(2):101–115. https://doi.org/10.1038/s41571-020-0422-y
Agyingi E, Wakabayashi L, Wiandt T et al (2018) Eden model simulation of re-epithelialization and angiogenesis of an epidermal wound. Processes 6:207. https://doi.org/10.3390/PR6110207
Sun R, Hu Z, Sottoriva A et al (2017) Between-region genetic divergence reflects the mode and tempo of tumor evolution. Nat Genet 49:1015–1024. https://doi.org/10.1038/NG.3891
Sottoriva A, Kang H, Ma Z et al (2015) A Big Bang model of human colorectal tumor growth. Nat Genet 47(3):209–216. https://doi.org/10.1038/ng.3214
Tubiana M (1989) Tumor cell proliferation kinetics and tumor growth rate. Acta Oncol 28:113–121. https://doi.org/10.3109/02841868909111193
Sottoriva A, Spiteri I, Shibata D et al (2013) Single-molecule genomic data delineate patient-specific tumor profiles and cancer stem cell organization. Cancer Res 73:41–49. https://doi.org/10.1158/0008-5472.CAN-12-2273
Rew DA, Wilson GD, Taylor I et al (1991) Proliferation characteristics of human colorectal carcinomas measured in vivo. Br J Surg 78:60–66. https://doi.org/10.1002/BJS.1800780120
Friesen DE, Baracos VE, Tuszynski JA (2015) Modeling the energetic cost of cancer as a result of altered energy metabolism: Implications for cachexia. Theor Biol Med Model 12:1–18. https://doi.org/10.1186/S12976-015-0015-0/FIGURES/7
Chen X, Chen S, Yu D (2020) Metabolic reprogramming of chemoresistant cancer cells and the potential significance of metabolic regulation in the reversal of cancer chemoresistance. Metabolites 10:1–15. https://doi.org/10.3390/METABO10070289
Strobl MAR, West J, Viossat Y et al (2021) Turnover modulates the need for a cost of resistance in adaptive therapy. Cancer Res 81:1135. https://doi.org/10.1158/0008-5472.CAN-20-0806
Li B, Brady SW, Ma X et al (2020) Therapy-induced mutations drive the genomic landscape of relapsed acute lymphoblastic leukemia. Blood 135:41. https://doi.org/10.1182/BLOOD.2019002220
Kim BJ, Forbes NS (2008) Single cell analysis demonstrates how nutrient deprivation creates apoptotic and quiescent cell populations in tumor cylindroids. Biotechnol Bioeng 101:797. https://doi.org/10.1002/BIT.21985
Acknowledgements
There is no funding source for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Video 1: Cell growth dynamics in glands at fission growth under adaptive therapy at \({g}_{r}={0.8g}_{s}\). (AVI 1749 KB)
Supplementary Video 2: Cell growth dynamics in glands at fission growth under adaptive therapy at \({g}_{r}={g}_{s}\). (AVI 574 KB)
Supplementary Video 3: Cell growth dynamics in glands at invasive growth under adaptive therapy at \({g}_{r}=0.8{g}_{s}\). (AVI 1188 KB)
Supplementary Video 4: Cell growth dynamics in glands at invasive growth under adaptive therapy at \({g}_{r}={g}_{s}\). (AVI 3628 KB)
Supplementary Video 5: Cell growth dynamics in glands in two clusters with \({l}_{sep}=10\) at invasive growth under adaptive therapy. (AVI 3699 KB)
Supplementary Video 6: Cell growth dynamics in glands in two clusters with \({l}_{sep}=40\) at invasive growth under adaptive therapy (AVI 2931 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, R.Z. Tumour Growth Mechanisms Determine Effectiveness of Adaptive Therapy in Glandular Tumours. Interdiscip Sci Comput Life Sci 16, 73–90 (2024). https://doi.org/10.1007/s12539-023-00586-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-023-00586-8