Abstract
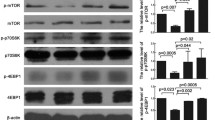
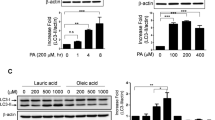
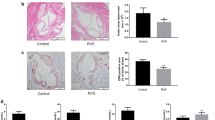
Cardiovascular disease due to atherosclerosis is one of the leading causes of death worldwide; however, the underlying mechanism has yet to be defined. The sodium-dependent glucose transporter 2 inhibitor (SGLT2i) empagliflozin is a new type of hypoglycemic drug. Recent studies have shown that empagliflozin not only reduces high glucose levels but also exerts cardiovascular-protective effects and slows the process of atherosclerosis. The purpose of this study was to elucidate the mechanism by which empagliflozin ameliorates atherosclerosis. Male apolipoprotein E-deficient (ApoE−/−) mice were fed a high-fat Western diet to establish an atherosclerosis model. The area and size of atherosclerotic lesions in ApoE−/− mice were then assessed by performing hematoxylin–eosin (HE) staining after empagliflozin treatment. Concurrently, oxidized low-density lipoprotein (oxLDL) was used to mimic atherosclerosis in three different types of cells. Then, following empagliflozin treatment of macrophage cells (RAW264.7), human aortic smooth muscle cells (HASMCs), and human umbilical vein endothelial cells (HUVECs), western blotting was applied to measure the levels of autophagy-related proteins and proinflammatory cytokines, and green fluorescent protein (GFP)-light chain 3 (LC3) puncta were detected using confocal microscopy to confirm autophagosome formation. Oil Red O staining was performed to detect the foaming of macrophages and HASMCs, and flow cytometry was used for the cell cycle analysis. 5-ethynyl-2′-deoxyuridine (EdU), cell counting kit-8 (CCK-8), and scratch assays were also performed to examine the proliferation and migration of HASMCs. Empagliflozin suppressed the progression of atherosclerotic lesions in ApoE−/− mice. Empagliflozin also induced autophagy in RAW246.7 cells, HASMCs, and HUVECs via the adenosine monophosphate–activated protein kinase (AMPK) signaling pathway, and it significantly increased the levels of the Beclin1 protein, the LC3B-II/I ratio, and p-AMPK protein. In addition, empagliflozin decreased the expression of P62 and the protein levels of inflammatory cytokines, and it inhibited the foaming of RAW246.7 cells and HASMCs, as well as the expression of inflammatory factors by inducing autophagy. Empagliflozin activated autophagy through the AMPK signaling pathway to delay the progression of atherosclerosis. Furthermore, the results of flow cytometry, EdU assays, CCK-8 cell viability assays, and scratch assays indicated that empagliflozin blocked HASMCs proliferation and migration. Empagliflozin activates autophagy through the AMPK signaling pathway to delay the evolution of atherosclerosis, indicating that it may represent a new and effective drug for the clinical treatment of atherosclerosis.






Similar content being viewed by others
Data availability
All data and materials involved in this work are available.
Abbreviations
- AS :
-
Atherosclerosis
- EMPA :
-
Empagliflozin
- SGLT2i :
-
Sodium-dependent glucose transporter 2 inhibitor
- HUVECs :
-
Human umbilical vein endothelial cells
- HUSMCs :
-
Human aortic smooth muscle cells
- RAW 264.7 :
-
A mouse macrophage line
- GFP-LC3 :
-
Green fluorescent protein-light chain 3
- oxLDL :
-
Human oxidized low-density lipoprotein
- AMPK :
-
Adenosine monophosphate-activated protein kinase
- T2DM :
-
Type 2 diabetes mellitus
- TNF-α :
-
Tumor necrosis factor-α
- IL-6 :
-
Interleukin-6
- mTORC1 :
-
Mammalian target of rapamycin complex 1
- ASCVD :
-
Atherosclerotic cardiovascular disease
References
Alloza I, Goikuria H, Freijo MDM, Vandenbroeck K (2016) A role for autophagy in carotid atherosclerosis. Eur Stroke J 1(4):255–263. https://doi.org/10.1177/2396987316674085
Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B (2020) Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 9(3):e014908. https://doi.org/10.1161/jaha.119.014908
Aviram M (2011) Atherosclerosis: cell biology and lipoproteins–inflammation and oxidative stress in atherogenesis: protective role for paraoxonases. Curr Opin Lipidol 22(3):243–4. https://doi.org/10.1097/MOL.0b013e3283474beb
Behnammanesh G, Durante GL, Khanna YP, Peyton KJ, Durante W (2020) Canagliflozin inhibits vascular smooth muscle cell proliferation and migration: role of heme oxygenase-1. Redox Biol 32:101527. https://doi.org/10.1016/j.redox.2020.101527
Cai C, Guo Z, Chang X, Li Z, Wu F, He J, Cao T, Wang K, Shi N, Zhou H, Toan S, Muid D, Tan Y (2022) Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKα1/ULK1/FUNDC1/mitophagy pathway. Redox Biol 52:102288. https://doi.org/10.1016/j.redox.2022.102288
Cai J, Zhang M, Liu Y, Li H, Shang L, Xu T, Chen Z, Wang F, Qiao T, Li K (2020) Iron accumulation in macrophages promotes the formation of foam cells and development of atherosclerosis. Cell Biosci 10(1):137. https://doi.org/10.1186/s13578-020-00500-5
De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM, Martinet W (2015) Autophagy in vascular disease. Circ Res 116(3):468–79. https://doi.org/10.1161/circresaha.116.303804
Di Pietro N, Formoso G, Pandolfi A (2016) Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc Pharmacol 84:1–7. https://doi.org/10.1016/j.vph.2016.05.013
Fei Y, Tsoi MF, Cheung BMY (2019) Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol 18(1):112. https://doi.org/10.1186/s12933-019-0916-z
Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S (2017) The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (-/-) mice fed a western diet. Diabetologia 60(2):364–376. https://doi.org/10.1007/s00125-016-4158-2
Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, Xu S, Xu Y, Hwa J, Li J, Shang H, Xiang Y (2021) Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. https://doi.org/10.1007/s13238-020-00809-4
Kasikara C, Doran AC, Cai B, Tabas I (2018) The role of non-resolving inflammation in atherosclerosis. J Clin Investig 128(7):2713–2723. https://doi.org/10.1172/jci97950
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412. https://doi.org/10.1371/journal.pbio.1000412
Lee H-H, Paudel KJ, Kim D-W (2015) Terminalia chebula fructus inhibits migration and proliferation of vascular smooth muscle cells and production of inflammatory mediators in RAW 2647. Evid Based Complement Alternat Med 2015:502182. https://doi.org/10.1155/2015/502182
Levine MJ (2017) Empagliflozin for type 2 diabetes mellitus: an overview of phase 3 clinical trials. Curr Diabetes Rev 13(4):405–423. https://doi.org/10.2174/1573399812666160613113556
Liu Y, Wu M, Xu B, Kang L (2021) Empagliflozin alleviates atherosclerosis progression by inhibiting inflammation and sympathetic activity in a normoglycemic mouse model. J Inflamm Res 14:2277–2287. https://doi.org/10.2147/jir.S309427
Liu Y, Xu J, Wu M, Xu B, Kang L (2021) Empagliflozin protects against atherosclerosis progression by modulating lipid profiles and sympathetic activity. Lipids Health Dis 20(1):5. https://doi.org/10.1186/s12944-021-01430-y
Lu YH, Chang YP, Li T, Han F, Li CJ, Li XY, Xue M, Cheng Y, Meng ZY, Han Z, Sun B, Chen LM (2020) Empagliflozin attenuates hyperuricemia by upregulation of ABCG2 via AMPK/AKT/CREB signaling pathway in type 2 diabetic mice. Int J Biol Sci 16(3):529–542. https://doi.org/10.7150/ijbs.33007
Martinet W, De Loof H, De Meyer GRY (2014) mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 233(2):601–607. https://doi.org/10.1016/j.atherosclerosis.2014.01.040
Meng Z, Liu X, Li T, Fang T, Cheng Y, Han L, Sun B, Chen L (2021) The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int Immunopharm 94:107492. https://doi.org/10.1016/j.intimp.2021.107492
Menghini R, Casagrande V, Marino A, Marchetti V, Cardellini M, Stoehr R, Rizza S, Martelli E, Greco S, Mauriello A, Ippoliti A, Martelli F, Lauro R, Federici M (2014) MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis 5(1):e1029. https://doi.org/10.1038/cddis.2013.556
Mewton N, Thibault H, Roubille F, Lairez O, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Cung TT, Finet G, Angoulvant D, Revel D, Bonnefoy-Cudraz E, Elbaz M, Piot C, Sahraoui I, Croisille P, Ovize M (2013) Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol 108(6):383. https://doi.org/10.1007/s00395-013-0383-8
Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E (2021) Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE((-/-)) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci 22(2). https://doi.org/10.3390/ijms22020818
Okamoto A, Yokokawa H, Sanada H, Naito T (2016) Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D 16(3):255–261. https://doi.org/10.1007/s40268-016-0137-9
Packer M (2020) SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 43(3):508–511. https://doi.org/10.2337/dci19-0074
Parahuleva MS, Lipps C, Parviz B, Hölschermann H, Schieffer B, Schulz R, Euler G (2018) MicroRNA expression profile of human advanced coronary atherosclerotic plaques. Sci Rep 8(1):7823. https://doi.org/10.1038/s41598-018-25690-4
Peyton KJ, Yu Y, Yates B, Shebib AR, Liu XM, Wang H, Durante W (2011) Compound C inhibits vascular smooth muscle cell proliferation and migration in an AMP-activated protein kinase-independent fashion. J Pharmacol Exp Ther 338(2):476–84. https://doi.org/10.1124/jpet.111.181784
Ren C, Sun K, Zhang Y, Hu Y, Hu B, Zhao J, He Z, Ding R, Wang W, Liang C (2021) Sodium-Glucose cotransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK-mTOR signaling pathway-mediated autophagy. Front Pharmacol 12:664181. https://doi.org/10.3389/fphar.2021.664181
Scannapieco FA, Bush RB, Paju S (2003) Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol 8(1):38–53. https://doi.org/10.1902/annals.2003.8.1.38
Schrijvers DM, De Meyer GR, Martinet W (2011) Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 31(12):2787–91. https://doi.org/10.1161/atvbaha.111.224899
Shadab M, Millar MW, Slavin SA, Leonard A, Fazal F, Rahman A (2020) Autophagy protein ATG7 is a critical regulator of endothelial cell inflammation and permeability. Sci Rep 10(1):13708. https://doi.org/10.1038/s41598-020-70126-7
Shao BZ, Han BZ, Zeng YX, Su DF, Liu C (2016) The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol Sin 37(2):150–6. https://doi.org/10.1038/aps.2015.87
Soltani A, Salmaninejad A, Jalili-Nik M, Soleimani A, Javid H, Hashemy SI, Sahebkar A (2019) 5’-Adenosine monophosphate-activated protein kinase: a potential target for disease prevention by curcumin. J Cell Physiol 234(3):2241–2251. https://doi.org/10.1002/jcp.27192
Tian J, Popal MS, Liu Y, Gao R, Lyu S, Chen K, Liu Y (2019) Ginkgo biloba leaf extract attenuates atherosclerosis in streptozotocin-induced diabetic ApoE-/- mice by inhibiting endoplasmic reticulum stress via restoration of autophagy through the mTOR signaling pathway. Oxid Med Cell Longev 2019:8134678. https://doi.org/10.1155/2019/8134678
Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai L, Chen Y, Zhao S, Fan J, Cheng X, Liu E (2016) Protein inhibitor of activated STAT3 suppresses oxidized ldl-induced cell responses during atherosclerosis in apolipoprotein E-deficient Mice. Sci Rep 6:36790. https://doi.org/10.1038/srep36790
Wright EM, Turk E (2004) The sodium/glucose cotransport family SLC5. Pflugers Archiv : Eur J Physiol 447(5):510–8. https://doi.org/10.1007/s00424-003-1063-6
Wright EM (2001) Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 280(1):F10-8. https://doi.org/10.1152/ajprenal.2001.280.1.F10
Wu M, Yang S, Wang S, Cao Y, Zhao R, Li X, Xing Y, Liu L (2020) Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front Pharmacol 11:223. https://doi.org/10.3389/fphar.2020.00223
Wu Z, Chen C, Luo J, Davis J, Zhang B, Tang L, Shi W, Liao D (2020) EGFP-EGF1-conjugated poly (lactic-co-glycolic acid) nanoparticles as a carrier for the delivery of CCR2- shRNA to atherosclerotic macrophage in vitro. Sci Rep 10(1):19636. https://doi.org/10.1038/s41598-020-76416-4
Xu J, Hirai T, Koya D, Kitada M (2021) Effects of SGLT2 inhibitors on atherosclerosis: lessons from cardiovascular clinical outcomes in type 2 diabetic patients and basic researches. J Clin Med 11(1). https://doi.org/10.3390/jcm11010137
Xu K, Yang Y, Yan M, Zhan J, Fu X, Zheng X (2010) Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J Lipid Res 51(9):2581–90. https://doi.org/10.1194/jlr.M005702
Zelniker TA, Braunwald E (2018) Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol 72(15):1845–1855. https://doi.org/10.1016/j.jacc.2018.06.040
Zhai C, Cheng J, Mujahid H, Wang H, Kong J, Yin Y, Li J, Zhang Y, Ji X, Chen W (2014) Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PloS One 9(3):e90563. https://doi.org/10.1371/journal.pone.0090563
Zhang Y, Wu Z, Yu H, Wang H, Liu G, Wang S, Ji X (2019) Chinese herbal medicine Wenxia Changfu formula reverses cell adhesion-mediated drug resistance via the integrin β1-PI3K-AKT pathway in lung cancer. J Cancer 10(2):293–304. https://doi.org/10.7150/jca.25163
Funding
This study was financially supported by the Scientific and Technological Department of Hubei Province (NO.2022CFA036), the National Natural Science Foundation of China (NO. 81771522), Hubei Provincial Health Commission Project (NO. WJ2021M258) and Cultivating Project for Young Scholars at Hubei University of Medicine (No. 2020QDJZR026).
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used. The authors’ responsibilities were as follows: HLX performed the experiments, analyzed the data, prepared the figures and wrote the manuscript; JF, QT, QYS and YZC provided support for experimental techniques and analyzed the data; ZC and FYW conceived the study, designed the experiments and helped with data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This experiment was approved by the Animal Management Committee of Hubei University of Medicine in accordance with the Animal Laboratory Animal Guidelines of Hubei University of Medicine and strictly abided by the rules of the Experimental Animal Ethics Committee of Hubei Medical College.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Empagliflozin reduces the area of atherosclerotic plaques in ApoE−/− mice.
• Empagliflozin induces autophagy in RAW246.7 cells, HASMCs and HUVECs.
• Empagliflozin may slow the progression of atherosclerosis by inducing autophagy.
• Empagliflozin may induce autophagy by activating the AMPK signaling pathway.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, H., Fu, J., Tu, Q. et al. The SGLT2 inhibitor empagliflozin attenuates atherosclerosis progression by inducing autophagy. J Physiol Biochem 80, 27–39 (2024). https://doi.org/10.1007/s13105-023-00974-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00974-0