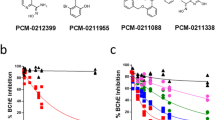
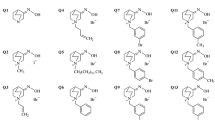
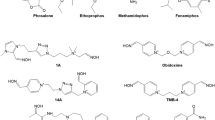
Abstract
Owing to their potential to cause serious adverse health effects, significant efforts have been made to develop antidotes for organophosphate (OP) anticholinesterases, such as nerve agents. To be optimally effective, antidotes must not only reactivate inhibited target enzymes, but also have the ability to cross the blood–brain barrier (BBB). Progress has been made toward brain-penetrating acetylcholinesterase reactivators through the development of a new group of substituted phenoxyalkyl pyridinium oximes. To help in the selection and prioritization of compounds for future synthesis and testing within this class of chemicals, and to identify candidate broad-spectrum molecules, an in silico framework was developed to systematically generate structures and screen them for reactivation efficacy and BBB penetration potential.









Similar content being viewed by others
Data availability
The supporting in vitro experimental data, SMILES representations of the target compounds, Structural characteristics of the target compounds, and other information related to the models and their verification are available in the Supplementary Material.
References
Tucker J (2007) War of nerves: chemical warfare from World War I to Al-Qaeda. Reprint edition. Anchor, New York
Costanzi S, Machado J-H, Mitchell M (2018) Nerve agents: what they are, how they work, how to counter them. ACS Chem Neurosci 9(5):873–885
King AM, Aaron CK (2015) Organophosphate and carbamate poisoning. Emerg Med Clin 33(1):133–151
Clement J (1979) Efficacy of pro-PAM (n-methyl-1, 6-dihydropyridine-2-carbaldoxime hydrochloride) as a prophylaxis against organophosphate poisoning. Toxicol Appl Pharmacol 47(2):305–311
Kuca K, Cabal J, Jun D, Kassa J, Bartosova L, Kunesova G, Dohnal V (2005) Strategy for the development of new acetylcholinesterase reactivators—antidotes used for treatment of nerve agent poisonings. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149(2):429–31
Skovira JW, O’Donnell JC, Koplovitz I, Kan RK, McDonough JH, Shih T-M (2010) Reactivation of brain acetylcholinesterase by monoisonitrosoacetone increases the therapeutic efficacy against nerve agents in Guinea pigs. Chemico-biol interact 187(1–3):318–324
Moshiri M, Darchini-Maragheh E, Balali-Mood M (2012) Advances in toxicology and medical treatment of chemical warfare nerve agents. DARU J Pharm Sci 20:1–24
Chambers JE, Meek EC, Chambers HW (2016) Novel brain-penetrating oximes for reactivation of cholinesterase inhibited by sarin and VX surrogates. Ann NY Acad Sci 1374(1):52–58
Chambers JE, Chambers HW, Funck KE, Meek EC, Pringle RB, Ross MK (2016) Efficacy of novel phenoxyalkyl pyridinium oximes as brain-penetrating reactivators of cholinesterase inhibited by surrogates of sarin and VX. Chemico-Biol Interact 259(Pt B):154–159. https://doi.org/10.1016/j.cbi.2016.07.004
Chambers JE, Chambers HW, Meek EC, Pringle RB (2013) Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates. Chemico-Biol Interact 203(1):135–138. https://doi.org/10.1016/j.cbi.2012.10.017
Chambers J, Wiygul S, Harkness J, Chambers H (1988) Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance-behavior in rats. Neurosci Res Commun 3(2):85–92
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Chambers JE, Chambers HW, Meek EC, Pringle RB (2013) Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates. Chemico-biol Interact 203(1):135–138
Wilson C, Cooper NJ, Briggs ME, Cooper AI, Adams DJ (2018) Investigating the breakdown of the nerve agent simulant methyl paraoxon and chemical warfare agents GB and VX using nitrogen containing bases. Org Biomol Chem 16(47):9285–9291
Chaubey K, Alam SI, Waghmare CK, Singh L, Srivastava N, Bhattacharya BK (2019) Differential proteome analysis of rat plasma after diisopropyl fluorophosphate (DFP) intoxication, a surrogate of nerve agent sarin. Chemico-Biol Interact 298:66–71
Meng F, Xi Y, Huang J, Ayers PW (2021) A curated diverse molecular database of blood–brain barrier permeability with chemical descriptors. Sci Data. https://doi.org/10.1038/s41597-021-01069-5
Wang W, Kim MT, Sedykh A, Zhu H (2015) Developing enhanced blood–brain barrier permeability models: integrating external bio-assay data in QSAR modeling. Pharm Res 32:3055–3065
Ooms F, Weber P, Carrupt P-A, Testa B (2002) A simple model to predict blood–brain barrier permeation from 3d molecular fields. Biochim Biophys Acta Mol Basis Dis 1587(2–3):118–125
Mensch J, Jaroskova L, Sanderson W, Melis A, Mackie C, Verreck G, Brewster ME, Augustijns P (2010) Application of Pampa-models to predict BBB permeability including efflux ratio, plasma protein binding and physicochemical parameters. Int J Pharm 395(1–2):182–197
Plisson F, Piggott AM (2019) Predicting blood–brain barrier permeability of marine-derived kinase inhibitors using ensemble classifiers reveals potential hits for neurodegenerative disorders. Mar Drugs 17(2):81
Zhao YH, Abraham MH, Ibrahim A, Fish PV, Cole S, Lewis ML, Groot MJ, Reynolds DP (2007) Predicting penetration across the blood–brain barrier from simple descriptors and fragmentation schemes. J Chem Inf Model 47(1):170–175
Li H, Yap CW, Ung CY, Xue Y, Cao ZW, Chen YZ (2005) Effect of selection of molecular descriptors on the prediction of blood–brain barrier penetrating and nonpenetrating agents by statistical learning methods. J Chem Inf Model 45(5):1376–1384
Landrum G (2013) Rdkit documentation. Release 1(1—-79):4
Thakur A, Patwa J, Sharma A, Flora SJ (2022) Synthesis, molecular docking, BSA, and in vitro reactivation study of imidazopyridine oximes against paraoxon inhibited acetylcholinesterase. Med Chem 18(2):273–287
Bhattacharjee KA, Musilek K, Kuca K (2013) In silico pharmacophore modeling on known pyridinium oxime reactivators of cyclosarin (GF) inhibited ache to aid discovery of potential, more efficacious novel non-oxime reactivators. Curr Comput Aided Drug Des 9(3):402–411
Bhattacharjee AK, Marek E, Le HT, Gordon RK (2012) Discovery of non-oxime reactivators using an in silico pharmacophore model of oxime reactivators of op-inhibited acetylcholinesterase. Eur J Med Chem 49:229–238
Bhattacharjee KA, Kuca K, Musilek K, Gordon KR (2012) An in silico stereo-electronic comparison of conventional pyridinium oximes and k-oximes for organophosphate (OP) poisoning. Med Chem 8(2):230–245
Lo R, Chandar NB, Kesharwani MK, Jain A, Ganguly B (2013) In silico studies in probing the role of kinetic and structural effects of different drugs for the reactivation of tabun-inhibited ache. PLoS ONE 8(12):79591
Petroianu G, Lorke D (2008) Pyridinium oxime reactivators of cholinesterase inhibited by diisopropyl-fluorophosphate (DFP): predictive value of in-vitro testing for in vivo efficacy. Mini Rev Med Chem 8(13):1328–1342
Lugokenski TH, Gubert P, Bueno DC, Nogara PA, Aquino Saraiva R, Barcelos RP, Carratu VS, Bresolin L, Vargas Barbosa NB, Pereira ME et al (2012) Effect of different oximes on rat and human cholinesterases inhibited by methamidophos: a comparative in vitro and in silico study. Basic Clin Pharmacol Toxicol 111(6):362–370
Ho TK (1995) Random decision forests. In: Proceedings of 3rd international conference on document analysis and recognition, vol 1, pp. 278–282. IEEE
Berrar D (2018) Bayes’ theorem and naive Bayes classifier. Encyclop Bioinform Comput Biol ABC Bioinform 403:412
Friedman JH (2001) Greedy function approximation: a gradient boosting machine. Ann Stat 29(5):1189–1232
Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, Ye Q, Liu T-Y (2017) LightGBM: a highly efficient gradient boosting decision tree. In: Advances in neural information processing systems, vol 30
Chen T, Guestrin C (2016) XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining, 2016, pp 785–794
Nembrini S, König IR, Wright MN (2018) The revival of the Gini importance? Bioinformatics 34(21):3711–3718
MacQueen I (1967) Some methods for classification and analysis of multivariate observations. In: Proceedings of the 5th Berkeley symposium on mathematical statistics problems, 1967, pp 281–297
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
Mukherjee S, Gupta RD (2020) Organophosphorus nerve agents: types, toxicity, and treatments. J Toxicol 2020:3007984. https://doi.org/10.1155/2020/3007984
Kumar R, Sharma A, Alexiou A, Bilgrami AL, Kamal MA, Ashraf GM (2022) DeePred-BBB: a blood brain barrier permeability prediction model with improved accuracy. Front Neurosci 16:858126
Yuan Y, Zheng F, Zhan C-G (2018) Improved prediction of blood–brain barrier permeability through machine learning with combined use of molecular property-based descriptors and fingerprints. AAPS J 20:1–10
Sakiyama H, Fukuda M, Okuno T (2021) Prediction of blood–brain barrier penetration (BBBP) based on molecular descriptors of the free-form and in-blood-form datasets. Molecules 26(24):7428
Chen B (2022) Retrosynthetic planning with retro*. Original-date: 2020-06-27T03:50:40Z. https://github.com/binghong-ml/retro_star. Accessed 12 Nov 2022
Chen B, Li C, Dai H, Song L (2020) Retro*: learning retrosynthetic planning with neural guided a* search. In: International conference on machine learning, 2020, pp 1608–1616. PMLR
Wang Z, Yang H, Wu Z, Wang T, Li W, Tang Y, Liu G (2018) In silico prediction of blood–brain barrier permeability of compounds by machine learning and resampling methods. ChemMedChem 13(20):2189–2201
Shaker B, Yu M-S, Song JS, Ahn S, Ryu JY, Oh K-S, Na D (2021) LightBBB: computational prediction model of blood–brain-barrier penetration based on lightGBM. Bioinformatics 37(8):1135–1139
Liu L, Zhang L, Feng H, Li S, Liu M, Zhao J, Liu H (2021) Prediction of the blood–brain barrier (BBB) permeability of chemicals based on machine-learning and ensemble methods. Chem Res Toxicol 34(6):1456–1467
Yu T-H, Su B-H, Battalora LC, Liu S, Tseng YJ (2022) Ensemble modeling with machine learning and deep learning to provide interpretable generalized rules for classifying CNS drugs with high prediction power. Brief Bioinform 23(1):377
Banks WA (2009) Characteristics of compounds that cross the blood–brain barrier. BMC Neurol 9(Suppl 1):3. https://doi.org/10.1186/1471-2377-9-S1-S3
Funding
Research presented in this publication was supported in part by the National Institutes of Health under Award Numbers U01NS083430 (JC), U01NS107127 (JC) and U01NS123255 (JC, EM). The views expressed in this publication are those of the authors and do not reflect the official policy or position of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
SH wrote the manuscript, developed the approach and mathematical models, conducted the simulations, and prepared the figures. JC and EM provided the experimental data and reviewed modeling results. BR wrote the manuscript, developed the methodology, reviewed all models and results, and provided supervision and resources. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habiballah, S., Chambers, J., Meek, E. et al. The in silico identification of novel broad-spectrum antidotes for poisoning by organophosphate anticholinesterases. J Comput Aided Mol Des 37, 755–764 (2023). https://doi.org/10.1007/s10822-023-00537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-023-00537-x