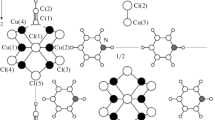
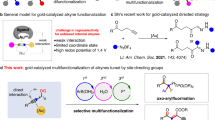
Abstract
Methods for carrying out coupling reactions in the chemistry of alkynes to form C–C bonds (oxidative dehydrocondensation reactions, Cadiot–Chodkiewicz reaction, and Sonogashira reaction) are analyzed and generalized. Protocols of synthesis of products of these reactions are also presented, including homogeneous and heterogeneous catalytic systems. In all cases, emphasis is placed on the kinetics and mechanisms of reactions with the discussion of the results of kinetic and spectrometric studies of the mechanisms of coupling reactions involving Cu(I, II, III), Au(I, III), Pd(0, I, II), and Fe (0, I, II, III) complexes. Particular attention is paid to the heterogeneous catalysis of oxidation reactions of alkynes with the participation of nanoparticles and nanoclusters of Pd, Au, Ag, and other metals. The nature of the intermediates containing these metals and the relationships between various oxidative and nonoxidative transformations of alkynes are discussed.


Similar content being viewed by others
Notes
Merkushev, E.B. and Shvartsberg, M.S., Iodine Organic Compounds and Syntheses Based on Them: A Tutorial, Tomsk: Tomsk. Gos. Pedagog. Inst. im. Leninskogo Komsomola, 1978.
Stang, P.J., Surber, B.W., Chen, Z.-C., Roberts, K.A., and Anderson A.G., Preparation and mechanism of formation of alkynyl tosylates and mesylates via tricoordinate iodonium species, J. Am. Chem. Soc., 1987, vol. 109, p. 228.
Archer, E.M. and Van Schalkwyk, T.G.D., The crystal structure of benzene iododichloride, Acta Cryst., 1953, vol. 6, p. 88.
Alcock, N.W., Countryman, R.M., Esperas, S., and Sawyer, J.F., Secondary bonding. Part 5.l. The crystal and molecular structures of phenyliodine(III) diacetate and bis(dichloroacetate), JCS Dalton Trans., 1979, p. 854.
REFERENCES
Temkin, O.N., “Golden Age” of homogeneous catalysis chemistry of alkynes: Dimerization and oligomerization of alkynes, Kinet. Catal., 2019, vol. 60, p. 689.
Temkin, O.N. and Bruk, L.G., The use of the concept of “oxidation state of an atom” and electronic balances in redox processes in organic and organoelement chemistry, Ross. Khim. Zh. (Zh. Ros. Khim. o-va im. D.I. Mendeleeva), 2014, vol. 58, nos. 5–6, p. 90.
Modern Alkyne Chemistry. Catalytic and Atom-Economic Transformations, Trost, B.M. and Li, Ch.-J., Eds., Weinheim: Wiley-VCH, 2015.
Stefanj, H.A., Guarezemini, A.S., and Cella, R., Tetrahedron, 2010, p. 7871.
Kotlyarovskii, I.L., Shvartsberg, M.S., and Fisher, L.B., in Reaktsii atsetilenovykh soedinenii (Reactions of acetylenic compounds), Novosibirsk: Nauka, 1967, p. 173.
Temkin, O.N. and Flid, R.M., Kataliticheskie prevrashcheniya atsetilenovykh soedinenii v rastvorakh kompleksov metallov (Catalytic Transformations of Acetylenic Compounds in Solutions of Metal Complexes), Moscow: Nauka, 1968.
Temkin, O.N., Shestakov, G.K., and Treger, Yu.A., Atsetilen. Khimiya. Mekhanizmy reaktsii. Tekhnologiya (Acetylene. Chemistry. Reaction Mechanisms. Technology), Moscow: Khimiya, 1991.
Lu, W. and Zhou, L., Oxidation of C–H Bonds, Wiley and Sons, 2017, ch. 8, p. 209.
Friis, S.D., Pirnot, M.T., Dupuis, L.N., and Buchwald, S.L., Angew. Chem., Int. Ed., 2017, vol. 56, p. 7242.
Kudryavtsev, Yu.P., Sladkov, A.M., Aseev, Yu.G., Nedoshivin, Yu.N., Kasatochkin, V.I., and Korshak, V.V., Dokl. Akad. Nauk SSSR, 1964, vol. 158, p. 389.
Eglinton, G. and Mc Grae, W., Advances in Organic Chemistry. Methods and Results, 1963, vol. 4, p. 225.
Sondheimer, F., Pure Appl. Chem., 1963, vol. 7, p. 363.
Bohlmann, F., in Chemistry of Acetylenes, Viehe, H.G., Ed., New York: M. Dekker, 1969, ch. 14, p. 977.
Siemsen, P., Livingston, R.C., and Diederich, F., Angew. Chem., Int. Ed., 2000, vol. 19, p. 2632.
Acetylene Chemistry: Chemistry, Biology, and Material Science, Diederich, F., Stang, P.J., and Tykwinski, R.R., Eds., Weinheim: Wiley-VCH, 2005.
Allen, S.E., Walvood, R.R., Padilla-Salinas, R., and Kozlowcki, M., Chem. Rev., 2013, vol. 113, p. 6234.
Zal’kind, Yu.S. and Fundyler, B.M., Zh. Org. Khim., 1936, vol. 6, p. 530.
Zal’kind, Yu.S. and Aizikovich, M.A., Zh. Org. Khim., 1937, vol. 7, p. 227.
Zal’kind, Yu.S. and Fundyler, B.M., Zh. Org. Khim., 1939, vol. 9, p. 1725.
Straus, F., Justus Liebigs Annalen der Chemie, 1905, vol. 342, no. 5, p. 190.
Akhtar, F., Richards, T.A., and Weedon, B.C.L., J. Chem. Soc., 1959, p. 933.
Balcioglu, N., Uraz, I., Bozkunt, C., and Sevin, F., Polyhedron, 1997, vol. 16, no. 2, p. 327.
Salkind, J.S. and Fundyler, B.M., Chem. Berichte, 1936, vol. 69, p. 128.
Zal’kind, Yu.S. and Gverdtsiteli, I.M., Zh. Org. Khim., 1939, vol. 9, p. 971.
Bowden, K., Heilbron, I., Jones, E.R.H., and Sargent, K.H., J. Chem. Soc., 1947, p. 1579.
Heilbron, I., Jones, E.R.H., and Sondheimer, F., J. Chem. Soc., 1947, p. 1586.
Zal’kind, Yu.S. and Kolyaskina, Z.N., Zh. Org. Khim., 1952, vol. 22, p. 2148.
Mkryan, G.M. and Papazyan, M.A., Dokl. Akad. Nauk Arm. SSR, 1953, vol. 16, p. 17.
Bohlmann, F. and Viehe, H.G., Chem. Berichte, 1954, vol. 87, p. 712.
Klebanskii, A.L., Grachev, I.V., and Kuznetsova, O.M., Zh. Org. Khim., 1957, vol. 27, p. 2977.
Bennett, E.G., Org. Chem., 1957, vol. 22, p. 557.
Copenhaver, J.W. and Bigelow, M.H., Acetylene and Carbon Monoxide Chemistry, New York: Reinhold, 1949, p. 121.
Reppe, W., Justus Liebigs Annalen der Chemie, 1955, vol. 596, p. 51.
Franke, W. and Meister, H., US Patent 2796442, 1957.
Hay, A.S., Org. Chem., 1960, vol. 25, p. 1275.
Hay, A.S., Org. Chem., 1962, vol. 27, p. 3320.
Eglinton, G. and Galbraith, A.R., Chem. Ind., 1956, p. 737.
Eglinton, G. and Galbraith, A.R., J. Chem. Soc., 1959, p. 889.
Sondheimer, F. and Amiel, Y.J., J. Am. Chem. Soc., 1956, vol. 78, p. 4178.
Sondheimer, F. and Amiel, Y., J. Am. Chem. Soc., 1957, vol. 79, p. 5817.
Sondheimer, F., Amiel, Y.J., and Wolovsky, R.J., J. Am. Chem. Soc., 1957, vol. 79, p. 6263.
Sondheimer, F., Wolovsky, R., and Ben-Efraim, D.A., J. Am. Chem. Soc., 1961, vol. 83, p. 1686.
Sondheimer, F. and Wolovsky, R.J., J. Am. Chem. Soc., 1962, vol. 84, p. 260.
Yadav, J.S., Reddy, B.V.S., Reddy, K.B., Uma, K., and Prasad, A.R., Tetrahedron Lett., 2003, vol. 44, p. 6493.
Li, Y.-N., Wang, J.-L., and He, L.-N., Tetrahedron Lett., 2011, vol. 52, p. 3485.
Wang, D., Li, J., Gao, T., Hou, S., and Chen, B., Green Chem., 2010, vol. 12, p. 45.
Adimurthy, S., Chandi, C., Malakar, C.C., and Beifuss, U., Org. Chem., 2009, vol. 74, p. 5648.
Zhu, M., Jin, J.C., and Tong, J.Y., J. Chem. Res., 2008, no. 4, p. 218.
Alcaide, B., Almendros, P., Carrascosa, R., and Rodriguez-Acehes, R., Eur. J. Org. Chem., 2008, p. 1375.
Balaraman, K. and Kesavan, V., Synthesis, 2010, no. 20, p. 3461.
Bedard, A.-C. and Collins, S.K., J. Am. Chem. Soc., 2011, vol. 133, p. 19976.
Bedard, A.-C. and Collins, S.K., Chem. Commun., 2012, vol. 48, p. 6420.
Godin, E., Bedard, A.-C., Raymond, M., and Collins, S.K., Org. Chem., 2017, vol. 82, p. 7576.
Clifford, O.A. and Waters, W., J. Chem. Soc., 1963, p. 3056.
Bohlmann, F., Schonowsky, H., Inhoffen, E., and Grau, G., Chem. Berichte, 1964, vol. 97, p. 94.
Fedenok, L.G., Berdnikov, V.M., and Shvartsberg, M.S., Zh. Org. Khim., 1973, vol. 9, p. 1781.
Fedenok, L.G., Berdnikov, V.M., and Shvartsberg, M.S., Zh. Org. Khim., 1975, vol. 11, p. 2492.
Fedenok, L.G., Berdnikov, V.M., and Shvartsberg, M.S., Zh. Org. Khim., 1974, vol. 10, p. 922.
Fedenok, L.G., Berdnikov, V.M., and Shvartsberg, M.S., Zh. Org. Khim., 1976, vol. 12, p. 1395.
Fedenok, L.G., Berdnikov, V.M., and Shvartsberg, M.S., Zh. Org. Khim., 1978, vol. 14, p. 1425.
Fedenok, L.G., Berdnikov, V.M., and Shvartsberg, M.S., Zh. Org. Khim., 1978, vol. 14, p. 1429.
Fedenok, L.G., Mechanism and synthetic possibilities of some reactions of acetylenic compounds, Doctor Sci. (Chem.) Dissertation, Novosibirsk: Inst. Khim. Kinet. Goreniya SO RAN, 2008.
Fedenok, L.G. and Shvartsberg, M.S., Tetrahedron Lett., vol. 52, p. 3776.
Tolman, W.B., Acc. Chem. Res., 1997, vol. 30, p. 227.
Holland, P. and Tolman, W.B., Coord. Chem. Rev., 1999, vols. 190–192, p. 855.
Khoan, Kh.M., Physical and chemical foundations of the catalytic synthesis of dialkynes, Cand. Sci. (Chem.) Dissertation, Moscow: Mosk. Inst. Tonkoi Khim. Teknol., 1990.
Khoan, Kh.M., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1994, vol. 35, no. 2, p. 266.
Khoan, Kh.M., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1994, vol. 35, no. 3, p. 367.
Sladkov, A.M., Gol’ding, I.R., Usp. Khim., 1979, vol. 10, no. 9, p. 1625.
Temkin, O.N., Homogeneous Catalysis with Metal Complexes. Kinetic Aspects and Mechanisms, Wiley, 2012.
Mykhalichko, B.M., Temkin, O.N., and Mys’kiv, M.G., Usp. Khim., 2000, vol. 69, no. 11, p. 1042.
Mc Connell, H.M. and Davidson, H., J. Am. Chem. Soc., 1950, vol. 72, no. 7, p. 3168.
Mc Connell, H.M. and Weaver, H.E., J. Chem. Phys., 1956, vol. 25, no. 2, p. 304.
Wilson, E.E., Oliver, A.G., Hughes, R.P., and Ashfeld, B.L., Organometallics, 2011, vol. 30, p. 5214.
Secutowski, D.G. and Stucky, G.D., J. Am. Chem. Soc., 1976, vol. 98, p. 1376.
Erker, G., Angew. Chem., 1986, vol. 98, p. 456.
Rosenthal, U. and Görls, H., J. Organomet. Chem., 1992, vol. 439, p. C36.
Rosenthal, U., Ohff, A., Tillack, A., and Baumann, W., J. Organomet. Chem., 1994, vol. 468, p. C4.
Pellny, P.-M., Burlakov, V.V., Peulecke, N., Spannenberg, A., Kempe, R., and Rosenthal, U., J. Organomet. Chem., 1999, vol. 578, p. 135.
Burlakov, V.V., Acetylene complexes of metallocenes of group IV B (4), Doctor Sci. (Chem.) Dissertation, Moscow: INEOS im. A.N. Nesmeyanova, 2012.
Wendlandt, A.E., Suess, A.M., and Stahl, S.S., Angew. Chem., Int. Ed., 2011, vol. 50, p. 11062.
Rosenthal, U., Ohff, A., Baumann, W., Kempe, R., Tillack, A., and Burlakov, V.V., Organometallics, 1994, vol. 13, p. 2903.
Rosenthal, U., Pubst, S., Arndt, P., Ohff, A., Tillack, A., Baumann, W., Kempe, R., and Burlakov, V.V., Organometallics, 1995, vol. 14, p. 2961.
Pubst, S., Arndt, P., Heller, B., Baumann, W., Kempe, R., and Rosenthal, U., Angew. Chem., Int. Ed., 1996, vol. 35, no. 10, p. 2454.
Heeres, H.J., Nijhoff, J., and Teuben, J.H., Organometallics, 1993, vol. 12, p. 2609.
Choukroun, R. and Cassoux, P., Acc. Chem. Res., 1999, vol. 32, p. 494.
Zhao, J., Zhang, S., Zhang, W.-X., and Xi, Z., Coord. Chem. Rev., 2014, vols. 270–271, p. 2.
Lttenauer, M.S., Mobian, P., and Barloy, L., Coord. Chem. Rev., 2022, vol. 459, p. 214.
Evans, W.J., Keyer, P.A., and Ziller, J.W., Organometallics, 1993, vol. 12, no. 7, p. 2618.
Khoan, Kh.M., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1994, vol. 35, no. 6, p. 889.
Coates, G.E. and Parkin, C., J. Inorg. Nucl. Chem., 1961, vol. 22, p. 59.
Corfield, P.W.R. and Shearer, H.M.M., Abst. Am. Cryst. Assoc. Meeting. Bozeman. Mont., 1964, p. 96.
Blake, D., Calvin, G., and Coates, G.E., Proc. Chem. Soc., 1959, p. 396.
Organocopper Compounds, Gmelin Handbook of Inorganic Chemistry, 8th ed., Berlin: Springer, 1986, vol. 60, p. 3.
Bedford, R.R., Hill, A.F., Thompsett, A.R., White, A.J.P., and Williams, D.J., Chem. Commun., 1996, p. 1059.
Collman, J.P. and Kang, J.W., J. Am. Chem. Soc., 1967, vol. 89, p. 844.
Nast, R. and Pfab, W., Chem. Berichte, 1956, vol. 89, p. 415.
Negishi, E., J. Am. Chem. Soc., 1991, vol. 113, p. 1440.
Kamata, K., Yamaguchi, S., Kotani, H., Yamaguchi, K., and Mizuno, N., Angew. Chem., Int. Ed., 2008, vol. 47, p. 2407.
Yamaguchi, K., Kamata, K., Yamaguchi, S., Kotani, H., and Mizuno, N.J., J. Catal., 2008, vol. 258, p. 121.
Milane, P., Duboc, C., Marrot, J., Riviere, E., Dolberg, A., and Secheresse, E., Chem. Eur. J., 2006, vol. 12, p. 1950.
Chaudhuri, P. and Wieghardt, K., Prog. Inorg. Chem., 1987, vol. 35, p. 330.
Fomina, L., Vazquez, B., Tkatchouk, E., and Fomine, S., Tetrahedron, 2002, vol. 58, p. 6741.
Efremov, G.E., Bovyrina, E.A., Katsman, E.A., Shamsiev, R.S., and Temkin, O.N., Izv. Akad. Nauk, Ser. Khim., 2019, no. 7, p. 1366.
Vilhelmsen, M.H., Jensen, J., Tortzen, C.G., and Nilsen, M.B., Eur. J. Org. Chem., 2013, p. 701.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5518.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5526.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5531.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5535.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5538.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5542.
Heck, R.F., J. Am. Chem. Soc., 1968, vol. 90, p. 5546.
Heck, R.F., J. Am. Chem. Soc., 1969, vol. 91, p. 6707.
Temkin, O.N., Kaliya, O.L., Shestakov, G.K., and Flid, R.M., Dokl. Akad. Nauk SSSR, 1970, vol. 190, p. 398.
Heck, R.F., J. Am. Chem. Soc., 1972, vol. 94, p. 2712.
Heck, R.F. and Nolley, J.P., Org. Chem., 1972, vol. 37, p. 2320.
Dieck, H.A. and Heck, R.F., J. Am. Chem. Soc., 1974, vol. 96, p. 1133.
Mizoroki, T., Mori, K., and Ozaki, A., Bull. Chem. Soc. Jpn., 1971, vol. 44, p. 581.
Kaliya, O.L., Temkin, O.N., Kirchenkova, G.S., Smirnova, E.M., Kimel’fel’d, L.M., and Flid, R.M., Izv. Akad. Nauk SSSR, Ser. Khim., 1969, p. 2854.
Kaliya, O.L., Kirchenkova, G.S., and Temkin, O.N., Kinet. Katal., 1969, vol. 10, p. 1186.
Temkin, O.N., Kaliya, O.L., Shestakov, G.K., Brailovskii, S.M., Flid, R.M., and Aseeva, A.P., Kinet. Katal., 1970, vol. 11, p. 1592.
Cassar, L., J. Organomet. Chem., 1975, vol. 93, p. 253.
Diek, H.A. and Heck, R.F., J. Organomet. Chem., 1975, vol. 93, p. 259.
Sonogashira, K., Tohda, Y., and Hagihara, N., Tetrahedron Lett., 1975, no. 50, p. 4467.
Rossi, R., Carpita, A., Quirici, M.G., and Gandenzi, M.L., Tetrahedron, 1982, vol. 38, p. 631.
Liu, Q. and Burton, D.J., Tetrahedron Lett., 1997, vol. 38, no. 25, p. 4371.
Vlassa, M., Ciocan-Tarta, I., Margineanu, F., and Oprean, I., Tetrahedron, 1996, vol. 54, no. 4, p. 1337.
Cho, D.H., Lee, J.H., and Kim, B.H., Org. Chem., 1999, vol. 64, p. 8048.
Fairlamb, I.J.S., Bäuerlein, P.S., Marrison, L.R., and Dickinson, J.M., Chem. Commun., 2003, p. 632.
Batsanov, A.S., Collings, J.C., Fairlamb, I.J.S., Holland, J.P., Howard, A.K., Liu, Z., Mard, T.R., Parsons, A.C., Ward, R.M., and Zhu, J., Org. Chem., 2005, vol. 70, p. 703.
Mc Glacken, G.P. and Fairlamb, I.J.S., Eur. J. Org. Chem., 2009, p. 4011.
Li, J.-H., Liang, Y., and Zhang, X.-D., Tetrahedron, 2005, vol. 61, p. 1903.
Yin, W., He, C., Chen, M., Zhang, H., and Lei, A., Org. Lett., 2009, vol. 11, no. 3, p. 709.
Berry, D.H. and Eisenberg, R., Organometallics, 1987, vol. 6, p. 1796.
Afzal, D., Lenhert, P.G., and Lukenhart, C.M., J. Am. Chem. Soc., 1984, vol. 106, p. 3050.
Gonzalez-Arellano, C., Abad, A., Corma, A., Garsia, H., Iglesias, M., and Sanchez, F., Angew. Chem., Int. Ed., 2007, vol. 46, p. 1536.
Plenio, H., Angew. Chem., Int. Ed., 2008, vol. 47, p. 6954.
Gonzalez-Arellano, C., Corma, A., Iglesias, M., and Sanchez, F., Eur. J. Inorg. Chem., 2008, p. 1107.
Li, P., Wang, L., Wang, M., and You, F., Eur. J. Org. Chem., 2008, p. 5946.
Hopkinson, M.N., Ross, J.E., Gluffredi, G.T., Gee, A.D., and Gouverneur, V., Org. Lett., 2010, vol. 12, no. 21, p. 4904.
Wegner, H.A. and Auzias, M., Angew. Chem., Int. Ed., 2011, vol. 50, p. 8236.
Leyva-Perez, A., Domenech, A., Al-Resayes, S.I., and Corma, A., ACS Catal., 2012, vol. 3, p. 121.
Peng, H., Xi, Y., Ronagi, N., Dong, B., Akhmedov, N.G., and Shi, X., J. Am. Chem. Soc., 2014, vol. 136, p. 13174.
Leyva-Perez, A., Domenech-Carbo, A., and Corma, A., Nat. Commun., 2015, vol. 6, p. 6703.
Ye, X., Peng, H., Wei, C., Yuan, T., Wojtas, L., and Shi, X., Chemistry, 2018, vol. 1983.
Beaumont, S.K., Kyriakou, G., and Lambert, R.M., J. Am. Chem. Soc., 2010, vol. 132, no. 35, p. 12246.
Boronat, M., Combita, D., Concepcion, P., Corma, A., Hermenegildo Garcia, H., Juarez, R., Laursen, S., and De Dios Lopez-Castro, J., J. Phys. Chem. C, 2012, vol. 116, p. 24855.
Boronat, M., Laursen, S., Leyva-Perez, A., Oliver-Meseguer, J., Combita, D., and Corma, A., J. Catal., 2014, vol. 315, p. 6.
Vulhanova, B., Vaclavik, J., Artiglia, L., Ranocchiari, M., Togni, A., and Van Bokhoven, J.A., ACS Catal., 2017, no. 7, p. 3414.
Chen, Z., Shen, R., Chen, C., Li, J., and Li, Y., Chem. Commun., 2018, vol. 54, p. 13155.
Cadio, P. and Chodkiewicz, W., in Chemistry of Acetylenes, Viehe, H.G., Ed., New York: M. Dekker, 1969, p. 597.
Bruk, L.G., Brailovskii, S.M., Temkin, O.N., Flid, R.M., and Kostyushin, A.S., Zh. Org. Khim., 1974, vol. 10, no. 11, p. 2262.
Shchel’tsyn, L.V., Brailovskii, S.M., Murugova, U.Yu., and Temkin, O.N., Kinet. Katal., 1988, vol. 29, no. 5, p. 1044.
Shchel’tsyn, L.V., Brailovskii, S.M., and Temkin, O.N., Kinet. Katal., 1990, vol. 31, no. 6, p. 1361.
Wityak, J. and Chan, J.B., Synth. Commun., 1991, vol. 21, p. 977.
Elbaum, D., Nguyen, T.B., Jorgensen, W.L., and Schreiber, S.L., Tetrahedron, 1994, vol. 50, p. 1503.
Cai, C. and Vasella, A., Helv. Chim. Acta, 1995, vol. 78, p. 2053.
Damie, S.V., Seomoon, D., and Lee, P.H., Org. Chem., 2003, vol. 68, p. 7085.
Lefevre, G., Franc, G., Tlili, A., Adamo, G., Taillefer, M., Ciofini, I., and Jutand, A., Organometallics, 2012, vol. 31, no. 22, p. 7694.
Weng, Y., Cheng, B., He, C., and Lei, A., Angew. Chem., Int. Ed., 2012, vol. 51, no. 38, p. 9547.
Amatore, K., Blart, E., Genet, J.P., Jutand, A., Lenaire-Audoire, S., and Savignac, M., Org. Chem., 1995, vol. 60, p. P. 6829.
Banerjee, S. and Patil, N.T., Chem. Commun., 2017, vol. 53, p. 7937.
Li, Y., Xie, X., Sun, N., and Liu, Y., Angew. Chem., Int. Ed., 2017, vol. 56, p. 6994.
Liu, Y., Yang, Y., Zhu, R., Liu, C., and Zhang, D., Catal. Sci. Technol., 2019, vol. 9, no. 15, p. 4091.
Deprez, N.R. and Sanford, M.S., Inorg. Chem., 2007, vol. 48, p. 1924.
Sonogashira, K., in Metal-Catalyzed Cross-coupling Reactions, Diederich, F. and Stang, P.J., Eds., Weinheim: Wiley-VCH, 1998, p. 203.
Sonogashira, K., Alkynes synthesis, in Handbook of Organopalladium Chemistry for Organic Synthesis, Negishi E.-I., Ed., New York: Wiley, 2002, vol. 1, p. 493.
Chinchilla, R. and Najera, C., Chem. Rev., 2007, vol. 107, p. 874.
Chinchilla, R. and Najera, C., Chem. Soc. Rev., 2011, vol. 40, p. 5084.
Karak, M., Barbosa, L., and Hargaden, G.C., RSC Adv., 2014, vol. 4, p. 53442.
Cacchi, S., Synthesis, 1986, p. 320.
Balanta, A., Godard, C., and Claver, C., Chem. Soc. Rev., 2011, vol. 40, p. 4973.
Bumagin, N.A., Ponomarev, A.B., and Beletskaya, I.P., Izv. Akad. Nauk SSSR, Ser. Khim., 1984, no. 7, p. 1561.
Gelman, D. and Buchwald, S.L., Angew. Chem., Int. Ed., 2003, vol. 2, p. 5991.
Beauperin, M., Job, A., Cattey, H., Royer, S., Meunier, P., and Hierso, J.-C., Organometallics, 2010, vol. 29, p. 2815.
Okuro, K. and Furuune, M., Enna, M., Miura, M., and Nomura, M., Org. Chem., 1993, vol. 58, p. 4716.
Kang, S.K., Yoon, S.-K., and Kim, Y.-M., Org. Lett., 2001, vol. 3, p. 2697.
Kollhofer, A. and Plenio, H., Adv. Synth. Catal., 2005, vol. 347, p. 1295.
Negishi, E. and Anastasia, L., Chem. Rev., 2003, vol. 103, p. 1979.
Reina, A., Dang-Bao, T., Guerrero-Rios, I., and Gómez, M., Nanomaterials, 2021, vol. 11, p. 1891.
Farina, V., Krishnamurthy, V., and Scott, W.J., The Stille Reaction, New York: J. Wiley and Sons, 1998.
Milstein, D. and Stille, J.K., J. Am. Chem. Soc., 2007, vol. 129, p. 11340.
Miyaura, N. and Suzuki, A., Chem. Commun., 1979, p. 866.
Portnoy, M. and Milstein, D., Organometallics, 1994, vol. 13, p. 3465.
Casado, A.L. and Espinet, P., Organometallics, 1998, vol. 17, no. 5, p. 954.
Fitton, P. and Rick, E.A., J. Organomet. Chem., 1971, vol. 28, p. 287.
Amatore, C. and Jutand, A., J. Organomet. Chem., 1999, vol. 576, p. 254.
Amatore, C. and Jutand, A., Acc. Chem. Res., 2000, vol. 33, p. 314.
Beletskaya, I.P. and Cheprakov, A.V., Chem. Rev., 2000, vol. 100, p. 3009.
Kozuch, S. and Jutand, A., Organometallics, 2005, vol. 24, p. 2319.
Barrios-Landeros, F. and Hartvig, J.F., J. Am. Chem. Soc., 2005, vol. 127, p. 6944.
Kozuch, S. and Shai, S., J. Am. Chem. Soc., 2006, vol. 128, p. 3355.
Tougerti, S. and Jutand, A., Chem. Eur. J., 2007, vol. 13, p. 666.
Hue, L. and Liu, Z., Chem. Soc. Rev., 2010, vol. 39, p. 1692.
Heiden, M.R., Plenio, H., Immel, S., Burello, E., Ruthenberg, G., and Hoefsloot, H.C., Chem. Eur. J., 2008, vol. 14, p. 2857.
Shekhar, S., Riberg, P., Hartwig, J.F., Mathiew, J.S., Blackmond, D.G., Strieter, E.R., and Buchwald, S.L., J. Am. Chem. Soc., 2006, vol. 128, p. 3584.
Hartwig, J.F., Inorg. Chem., 2007, vol. 46, p. 1936.
Osakada, K., Takisawa, T., and Yamamoto, T., Organometallics, 1995, vol. 14, p. 3531.
Nova, A., Ujaque, G., Maseros, F., Ledos, A., and Espinet, P., J. Am. Chem. Soc., 2006, vol. 128, p. 14571.
Jutand, A., Negri, S., and Principaud, A., Eur. J. Inorg. Chem., 2005, p. 631.
Genet, J.P., Blart, E., and Savignac, M., Synlett, 1992, p. 715.
Alami, M., Ferri, F., and Linstrumelle, G., Tetrahedron Lett., 1993, vol. 34, no. 40, p. 6403.
Böhm, V.P.W. and Herrmann, W.A., Eur. J. Org. Chem., 2000, p. 3679.
Mery, D., Heuze, K., and Astruc, D., Chem. Commun., 2003, p. 1934.
Fukuyama, T., Shinmen, M., Nishitani, S., Sato, M., and Ryu, L., Org. Lett., 2002, vol. 4, no. 10, p. 1691.
Alonso, D.A., Najera, C., and Pacheco, C., Tetrahedron Lett., 2002, vol. 43, p. 9365.
Leadbeater, N.E. and Tominack, B.J., Tetrahedron Lett., 2003, vol. 44, p. 8653.
Urgaonkar, S. and Verkade, J.G., Org. Chem., 2004, vol. 69, p. 5752.
Mori, A., Kawoshima, J., Shimada, T., Suguro, M., Hirabayashi, K., and Nishihara, Y., Org. Lett., 2000, vol. 2, no. 19, p. 2935.
Iranpoor, N., Firouzabadi, H., and Ahmadi, Y., Eur. J. Org. Chem., 2012, p. 305.
Amatore, C., Bensalem, S., GHam, S., and Jutand, A., J. Organometall. Chem., 2004, vol. 689, p. 4642.
Ljungdahl, T., Bennur, T., Dallas, A., Emtenas, H., and Matensson, J., Organometallics, 2008, vol. 27, p. 2490.
Gacia-Meldhor, M., Pacheco, M.C., Najera, C., Ledos, A., and Ujaque, G., ACS Catal., 2012, vol. 2, p. 135.
De Souza, R.O.M.A., Bittar, M.S., Mendes, L.V.P., and Da Silva, C.M.F., Synlett, 2008, no. 12, p. 1777.
Kuriakou, G., Beaumont, S.K., Humphrey, S.M., Antonetti, C., and Lambert, R.M., ChemCatChem, 2010, vol. 2, p. 1444.
Suomin, D. and Koel, B.E., Surf. Sci., 2001, vol. 490, p. 265.
Kanuru, V., Kuriakow, G., Beaumont, S.K., Papageorgiu, A.C., Watson, D.J., and Lambert, R.M., J. Am. Chem. Soc., 2010, vol. 132, p. 8081.
Goguet, A., Ace, M., Saih, Y., Sa, J., Kavanagh, J., and Hardacre, C., Chem. Commun., 2009, p. 4889.
Lauterbach, T., Livendahl, M., Rosellon, A., Espinet, P., and Echavarren, A.M., Org. Lett., 2010, vol. 12, no. 13, p. 3006.
Corma, A., Juarez, R., Boronat, M., Sanchez, F., Iglesias, M., and Garsia, H., Chem. Commun., 2011, vol. 47, p. 1446.
Espinet, P. and Echavarren, A.M., Platinum Metals Rev., 2011, vol. 55, no. 3, p. 212.
Leuva-Perez, A., Oliver-Meseguer, J., Cabrero-Antonio, J.P., Rubio-Marques, R., Serna, P., Al-Resayes, S.I., and Corma, A., ACS Catal., 2013, vol. 3, no. 8, p. 1865.
Boronat, M., Lopes-Ausens, T., and Corma, A., J. Phys. Chem., 2014, vol. 118, no. 17, p. 9018.
Li, G. and Jiang, D., J. Catal., 2013, vol. 306, p. 177.
Robinson, P.S.D. and Khairallah, G.K., Da Silva, G., Lioe, H., and O’Hair, R.A.J., Angew. Chem., Int. Ed., 2012, vol. 51, p. 3812.
Nijamudheen, A. and Datta, A., J. Phys. Chem., 2013, vol. 117, p. 21433.
Lin, J., Abroshan, H., Liu, C., Zhu, M., Li, G., and Haruta, M., J. Catal., 2015, vol. 330, p. 354.
Johansson, N., Sisodiyas, S., Shyesteh, P., Chaudhary, S., Andersen, J.N., Knudsen, J., Wendt, O.F., and Schnadt, J., J. Phys. Condens. Matter, 2017, vol. 29, p. 444005.
Zeineddine, A., Estevez, L., Malet-Ladeira, S., Miquen, K., Amgoune, L., and Bourosson, D., Nat. Commun., 2017, vol. 8, no. 1, p. 565.
Jones, L.A., Sanz, S., and Laguna, M., Catal. Today, 2007, vol. 122, p. 403.
Panda, B. and Sarkar, T.K., Tetrahedron Lett., 2010, vol. 51, p. 301.
Panda, B. and Sarkar, T.K., Chem. Commun., 2010, vol. 46, p. 3131.
Hashmi, A.S.K., Lothschutz, C., Dopp, R., Rudolph, M., Ramamurthi, T.D., and Rominger, F., Angew. Chem., 2009, vol. 48, no. 44, p. 8243.
Liu, R., Chen, H., Fang, L., Xu, C., He, Z., Lai, Y., Zhao, H., Bekana, D., and Liu, J.-F., Environ. Sci. Technol., 2018, vol. 52, p. 4244.
Rossy, J., Majimel, J., Fouqeuet, E., Delacote, C., Boujita, M., Labrugere, C., Treguer-Dlapierre, M., and Felkin, F.-X., Chem. Eur. J., 2013, vol. 19, p. 14024.
Reina, A., Dang-Bao, T., Guerrero-Rios, I., and Gomez, M., Nanomaterials, 2021, vol. 11, p. 1891.
Zhu, M., Zhou, Z., and Chen, R., Synthesis, 2018, no. 17, p. 2680.
Venkatesan, P. and Santhanalakshmi, J., Langmuir, 2010, vol. 26, no. 14, p. 12225.
Weibel, J.-M., Blanc, A., and Pale, P., Chem. Rev., 2008, vol. 108, p. 3149.
Li, P.H. and Wang, L., Synlett, 2006, p. 2261.
Halbes-Letinois, H., Pale, P., and Berger, S., Org. Chem., 2005, vol. 70, p. 9185.
Jeffery, T., JCS Chem. Commun., 1991, p. 324.
Wu, H.-J., Hsu, H.-K., and Chiang, C.-M., J. Am. Chem. Soc., 1999, vol. 121, no. 18, p. 4433.
Han, M., Liu, S., Nie, X., Yuan, D., Sun, P., Dai, Z., and Bao, J., RSC Adv., 2012, vol. 2, p. 6061.
Sanches-Sanches, C., Orozco, N., Holgado, J.P., Beaumont, S.K., Kuriakou, G., Watson, D.J., Gonzalez-Elipe, A.R., Feria, L., Sanz, J.F., and Lambert, R.M., J. Am. Chem. Soc., 2015, vol. 137, p. 940.
Bumagin, N.A., Kalinovskii, I.O., and Ponamorev, A.B., Izv. Akad. Nauk SSSR, Ser. Khim., 1986, no. 12, p. 2836.
Beletskaya, I.P., Latyshev, G.V., Tsvetkov, A.V., and Lukashev, N.V., Tetrahedron Lett., 2003, vol. 44, p. 5011.
Vechorkin, O., Barmaz, D., Pronst, V., and Hu, X., J. Am. Chem. Soc., 2009, vol. 131, p. 12078.
Son, S.U., Jang, Y., Park, J., Bin, N.H., Park, H.M., Yun, H.J., Lee, J., and Hueon, T., J. Am. Chem. Soc., 2004, vol. 126, p. 5026.
Feng, L., Liu, F., Sun, P., and Bao, J., Synlett, 2008, no. 9, p. 1415.
Kanuru, V.K., Humphrey, S.M., Kyffi, J.M.W., Jefferson, D.A., Burton, J.W., Armbruster, M., and Lambert, R.M., Dalton Trans., 2009, p. 7602.
Enthaler, S., Junge, K., and Beller, M., Angew. Chem., Int. Ed., 2008, vol. 47, p. 3317.
Correa, A., Mancheno, O.G., and Bolm, C., Chem. Soc. Rev., 2008, vol. 37, p. 1108.
Bauer, I. and Knolker, H.J., Chem. Rev., 2015, vol. 115, pp. 3170–3387.
Carril, M., Correa, A., and Bolm, K., Angew. Chem., Int. Ed., 2008, vol. 47, p. 4862.
Correa, A., Elmore, S., and Bolm, K., Chem. Eur. J., 2008, vol. 14, p. 3527.
Bistri, O., Correa, A., and Bolm, K., Angew. Chem., Int. Ed., 2008, vol. 47, no. 3, p. 596.
Correa, A., Corril, M., and Bolm, K., Angew. Chem., Int. Ed., 2008, vol. 47, p. 2880.
Xie, X., Xu, X., Li, H., Xu, Y., Yang, J., and Li, Y., Adv. Synth. Catal., 2009, vol. 351, p. 1263.
Pan, C., Luo, F., Wang, W., Ye, Z., and Liu, M., J. Chem. Res., 2009, p. 478.
Hung, T.-T., Huang, C.-M., and Tsai, F.-Y., ChemCatChem, 2012, vol. 4, p. 540.
Yang, J., Shen, G., and Chen, D., Synth. Commun., 2013, vol. 43, p. 837.
Gruber, M., Chouzier, S., Koehler, K., and Djakovitch, L., Appl. Catal. A: Chem., 2004, vol. 265, p. 161.
Rao Volla, C.M. and Vogel, P., Tetrahedron Lett., 2008, vol. 49, p. 5961.
Mao, J., Xie, G., Wu, M., Guo, J., and Li, S., Adv. Synth. Catal., vol. 350, p. 2477.
Buchwald, S. and Bolm, C., Angew. Chem., Int. Ed., 2009, vol. 48, p. 5586.
Larsson, P.-F., Correa, A., Carril, M., Norrby, P.-O., and Bolm, C., Angew. Chem., Int. Ed., 2009, vol. 48, p. 5691.
Bedford, R.B., Nakamura, M., Gower, J., Haddow, M.F., Hall, M.A., Huwe, M., Hashimoto, T., and Okopie, R.A., Tetrahedron Lett., 2009, vol. 50, p. 6110.
Savant, D.N., Tambade, J., Wagh, S., and Bhanage, B.M., Tetrahedron Lett., 2010, vol. 51, p. 2758.
Hatakeyama, T., Yoshimoto, Y., Gabriel, T., and Nakamura, M., J. Org. Lett., 2008, vol. 10, no. 23, p. 5341.
Berben, L.A. and Long, J.R., Inorg. Chem., 2005, vol. 44, no. 23, p. 8459.
Furstner, A., Nartin, R., Krause, H., Seidel, G., Goddard, R., and Lehman, C.W., J. Am. Chem. Soc., 2008, vol. 130, p. 8773.
Hatakeyama, T., Hashimoto, S., Ishizuka, K., and Nakamura, M., J. Am. Chem. Soc., 2009, vol. 131, p. 11949.
Firouzabadi, H., Iranpoor, N., Gholinejad, M., and Hoseini, J., Adv. Synth. Catal., 2011, vol. 353, p. 125.
Park, S., Kim, M., Koo, D.H., and Cheng, S., Adv. Synth. Catal., 2004, vol. 346, p. 1638.
Na, Y., Park, S., Han, S.B., Han, H., Ko, S., and Chang, S., J. Am. Chem. Soc., 2004, vol. 126, no. 1, p. 250.
Borah, H.N., Prajapati, D., and Boruah, R.C., Synlett, 2005, no. 18, p. 2823.
Freiberg, M., Mulac, W.A., Schmidt, K.H., and Meyerstein, D., JCS Faraday I, 1980, vol. 76, p. 1838.
Freiberg, M., Meyerstein, D., and Yamamoto, Y., JCS. Dalton Trans., 1982, p. 1137.
Cohen, H. and Meyerstein, D., Inorg. Chem., 1986, vol. 25, p. 1505.
Masarawa, M., Cohen, H., and Meyerstein, D., Inorg. Chem., 1986, vol. 25, p. 4897.
Cohen, H. and Meyerstein, D., JCS Faraday I, 1988, vol. 84, no. 11, p. 4157.
Masarawa, M., Cohen, H., Glaser, R., and Meyerstein, D., Inorg. Chem., 1990, vol. 24, p. 5031.
Goldstein, S., Czapski, G., Cohen, H., and Meyerstein, D., Inorg. Chim. Acta, 1992, vol. 192, p. 87.
Navon, X., Golub, G., Cohen, H., and Meyerstein, D., Organometallics, 1995, vol. 14, p. 5670.
Szulc, A., Meyerstein, D., and Cohen, H., Inorg. Chim. Acta, 1998, vol. 270, p. 440.
Epstein, D.M., Cohen, H., Masarawa, A., and Meyerstein, D., Inorg. Chim. Acta, 2002, vol. 339, p. 281.
Burg, A. and Meyerstein, D., in Inorganic/Bioinorganic Reaction Mechanisms, 2012, vol. 64, p. 220.
Yardent, G., Meyerstein, D., Kats, L., Cohen, H., Zilberman, I., and Maimon, E., J. Coord. Chem., 2019, vol. 72, nos. 22–24, p. 3445.
Mykhalichko, I.M., Mys’kiv, M.G., and Davydov, V.N., Zh. Neorg. Khim., 1999, vol. 44, no. 1, p. 46.
Catalysis by Gold. Catalytic Science, Bond, G.C., Louis C., and Thompson, D.T., Eds., London: Imp. College Press, 2006, vol. 6.
Gorin, D.J., Toste, F.D., and Reina, A., Nature, 2007, vol. 446, p. 395.
Modern Gold Supramolecular Chemistry. Gold–Metal Interaction and Application, Laguna, A., Ed., Weinheim: Wiley-VCH, 2009.
Gold Chemistry: Applications and Future Directions in the Life Sciences, Mohr, F., Ed., Weinheim: Wiley-VCH, 2009, p. 408.
Soriano, E. and Marco-Conteeles, J., Top Curr. Chem., 2011, vol. 302, p. 1.
Zubanova, E.M., Reaction mechanisms of copper complexes with alkyl radicals, Extended Abstract of Cand. Sci. (Chem.) Dissertation, Moscow: Mosk. Gos. Univ. im. M.V. Lomonosova, 2015.
Modern Gold Catalyzed Synthesis, Hashmi, A.S.K. and Toste, F.D., Eds., Weinheim: Wiley-VCH, 2012.
Zang, L., Acc. Chem. Res., 2014, vol. 47, no. 3, p. 877.
Rosca, D.-A., Fernandez-Cestau, J., Huges, D.-L., and Bohmann, M., Organometallics, 2015, vol. 34, no. 11, p. 2098.
ACKNOWLEDGMENTS
I thank K. Egiazaryan, graduate student, Chair of Physical Chemistry, MIREA—Russian Technological University, Moscow, Russia, for his invaluable help in preparing this review; Dr. L.G. Fedenok for very useful discussions concerning the mechanisms of the OD reaction; Professor O.L. Kalija and Professor L.G. Bruk for valuable advice on the content and structure of the review.
Funding
No support was received for the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Abbreviations and notation: EDA, ethylenediamine; TMEDA, tetramethylethylenediamine; THF, tetrahydrofuran; MA, methylacetylene; DMDA, dimethyldiacetylene; CMA, chloromethylacetylene; DA, diacetylene; MDA, methyldiacetylene; DCE, dichloroethylene; PEG, polyethylene glycol; BMA, bromomethylacetylene; CMA, copper methylacetylide; TBA, tetrabutylamine; DMF, dimethylformamide; EG, ethylene glycol; Py, pyridine; PiPy, piperidine; DiPy, dipyridyl; AN, acetonitrile; Phen, phenanthrene; BMIM, butylmethylimidazole; dba, dibenzylideneacetone; hal, halides; dppm, 1,2-bis(diphenylphosphino)methane; dppp, 1,2-bis(diphenylphosphino)propane; dppe, 1,2-bis(diphenylphosphino)ethane; dppf, 1,1-bis(diphenylphosphino)ferrocene; BINAP, bis(naphthyldiphenylphosphine); Xantophos, 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene; TEMPO, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl; TMEDA, tetramethylethylenediamine; TMED, tetramethylethylenediamine; DME, dimethyl ether; DMED, dimethylethylenediamine; NMP, N-methyl-2-pyrrolidone; dipy, dipyridyl; NHC, N-heterocyclic carbene; HX, acids; D, optical density; POCC, polyfunctional oxygen-containing compounds; NMR, nuclear magnetic resonance; OS, oxidation state; ORR, redox reactions; OD, oxidative dehydrocondensation of alkynes (coupling); B, base; NC, nanocluster; NP, nanoparticle; OA, oxidative addition; TM, transmetalation; RE, reductive elimination; rt, room temperature; TOF, turnover frequency of catalyst; TON, turnover number of catalyst; EDG, electron donating group; EWG, electron withdrawing group; KIE, kinetic isotope effect; IL, ionic liquids; RDS, rate-determining step; TPR, temperature-programmed reaction; CSI-MS, cold-spray ionization mass spectrometry; STM, scanning tunneling microscopy; ESI-MS, electrospray ionization mass spectrometry; CID, collision-induced dissociation; XPS, X-ray photoelectron spectroscopy; HR TEM, high-resolution transmission electron microscopy, FE SEM, field emission scanning electron microscopy; STM, scanning tunneling microscopy; DFT, density functional theory; XRD, X-ray powder diffraction analysis; and ICP-ES, inductively coupled plasma emission spectroscopy.
Rights and permissions
About this article
Cite this article
Temkin, O.N. “Golden Age” of Homogeneous Catalytic Chemistry of Alkynes: Some Oxidative Transformations of Alkynes (A Review). Kinet Catal 64, 521–577 (2023). https://doi.org/10.1134/S0023158423050117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158423050117