Abstract
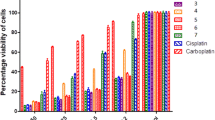
A series of σ-aryl platinum complexes with the sterically hindered phenol group of the general formula RPt[PPh3]2X (R = 3,5-di-tert-butyl-4-hydroxyphenyl; X = Cl (I), diclofenac (II), aspirin (III), and OOCR (IV)) is synthesized and characterized by 1H, 13C, and 31P NMR spectroscopy, IR spectroscopy, and elemental analysis. The molecular structure of compound I is determined by X-ray diffraction (XRD) (CIF file CCDC no. 2243100). The electron and hydrogen atom transfers are studied by spectrophotometry in the CUPRAC and DPPH tests. Complexes I, II, and IV are active reducing agents of Cu(II). The antioxidant activity is studied as the ability of the compounds to inhibit lipoxygenase (LOX-1B). Compound I is found to be an inhibitor of LOX-1B. The antiproliferative properties of the complexes are studied in vitro on the HCT-116, MCF-7, and A-549 cancer cells and WI-38 normal cells. The synthesized compounds have a lower antiproliferative activity than that of cisplatin.

Similar content being viewed by others
REFERENCES
Guddneppanavar, R., Saluta, G., Kucera, G.L., and Bierbach, U., J. Med. Chem., 2014, vol. 49, no. 11, p. 3204.
Kelland, L.R., Barnard, F.G.C., Mellish, K.J., and Jones, M., Cancer Res., 1994, vol. 8, p. 5618.
Cheng, Q., Wang, H., Min, Y., and Wang, J., Chem. Commun., 2014, vol. 50, p. 7427.
Hager, S., Ackermann, C.J., Joerger, M., et al., Annals Oncology, 2016, vol. 27, p. 975.
Intini, F.P., Zajac, J., Novohradsky, V., et al., Inorg. Chem., 2017, vol. 56, no. 3, p. 1483.
Ayoub, S.S., Botting, R.M., Joshi, A.N., et al., Mol. Cell. Biochem., 2009, vol. 327, nos. 1–2, p. 101.
D’Autreaux, B. and Toleda, M.B., Nat. Rev. Mol. Cell Biol., 2007, vol. 8, no. 10, p. 813.
Sies, H., Berndt, C., and Jones, D.P., Annu. Rev. Biochem., 2017, vol. 86, p. 715.
Antonenko, T.A., Shpakovsky, D.B., Vorobyov, M.A., et al., Appl. Organomet. Chem., 2018, vol. 32, р. e4381.
Antonenko, T.A., Gracheva, Yu.A., Shpakovsky, D.B., et al., Int. J. Mol. Sci., 2023, vol. 24, p. 2024.
Brauer, G., Handbook of Preparative Inorganic Chemistry, New York: Academic, 1983, vol. 5, р. 1814.
Milaev, A.G., Panov, V.B., and Okhlobystin, O.Yu., Zh. Obshch. Khim., 1981, vol. 51, no. 12, p. 2715.
Malatesta, L. and Cariello, C., J. Chem. Soc., 1958, no. 6, p. 2323.
Milaeva, E.R., Rubezhov, A.Z., Prokoph’ev, A.I., and Okhlobystin, O.Yu., J. Organomet. Chem., 1980, vol. 188, no. 3, p. 43.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Adv., 2015, vol. 71, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Apak, R., Guglu, K., Ozyurek, M., and Karademir, S., J. Agric. Food Chem., 2004, vol. 52, p. 7970.
Brand-Williams, W., Cuvelier, M.E., and Berset, C., Food Sci. Technol., 1995, vol. 28, p. 25.
Ozturk, I., Filimonova, S., and Hadjikakou, S.K., Inorg. Chem., 2010, no. 7, p. 488.
Niks, M. and Otto, M., J. Immunol. Methods, 1990, vol. 130, no. 1, p. 149.
Ustafa, M.O., Agric. Food Chem., 2004, vol. 52, p. 7970.
Funding
This work was supported by the Russian Science Foundation, project no. 22-63-00016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Antonenko, T.A., Shpakovskii, D.B., Gracheva, Y.A. et al. trans-Platinum Complexes with Diclofenac, Aspirin, and 2,6-Di-tert-Butylphenol Fragment: Synthesis and Biological Activity. Russ J Coord Chem 49, 652–659 (2023). https://doi.org/10.1134/S1070328423600511
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328423600511