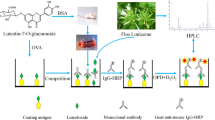
Abstract
Saikosaponins are naturally occurring oleanane-type triterpenoids that are found in Bupleuri radix (root of Bupleurum falcatum) and exhibit a broad biological activity spectrum. Saikosaponin b2 (SSb2) is the main saikosaponin in Kampo medicine extracts and is a designated quality control marker for the same in the Japanese Pharmacopeia. Although some monoclonal antibodies (mAbs) against saikosaponins have been produced to evaluate the quality of Bupleuri radix and related products, anti-SSb2 mAbs have not been used to quantify SSb2 in Kampo medicines. To address this knowledge gap, we herein established a new hybridoma cell line secreting a highly specific anti-SSb2 mAb and developed an indirect competitive enzyme-linked immunosorbent assay (icELISA) based on this mAb for the detection of SSb2 in Bupleuri radix-containing Kampo medicines. The generated SSb2-recognized mAb exhibited high specificity to SSb2 in icELISA. The developed assay featured high sensitivity (linearity range = 1.95–125 ng/ml), accuracy, precision and reproducibility (coefficient of variation < 5%), and the thus determined SSb2 contents were strongly correlated with those obtained using liquid chromatograph–mass spectrometer. These results suggest that the anti-SSb2 mAb-based icELISA method can be used for the quality control and standardization of Kampo medicines containing Bupleuri radix.



Similar content being viewed by others
References
Yang F, Dong X, Yin X, Wang W, You L, Ni J (2017) Radix Bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. BioMed Res Int 2017:7597596. https://doi.org/10.1155/2017/7597596
Li X, Li X, Huang N, Liu R, Sun R (2018) A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine 50:73–87. https://doi.org/10.1016/j.phymed.2018.09.174
Kim BM (2018) The role of saikosaponins in therapeutic strategies for age-related diseases. Oxid Med Cell Longev 2018:8275256. https://doi.org/10.1155/2018/8275256
Lee TH, Park S, You MH, Lim JH, Min SH, Kim BM (2016) A potential therapeutic effect of saikosaponin C as a novel dual-target anti-Alzheimer agent. J Neurochem 136:1232–1245. https://doi.org/10.1111/jnc.13515
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ, Liao M, Chen JX (2012) Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int Immunopharmacol 14:121–126. https://doi.org/10.1016/j.intimp.2012.06.010
Xu L, Su J, Guo L, Wang S, Deng X, Ma S (2019) Modulation of LPA1 receptor-mediated neuronal apoptosis by Saikosaponin-d: a target involved in depression. Neuropharmacology 155:150–161. https://doi.org/10.1016/j.neuropharm.2019.05.027
Li HY, Zhao YH, Zeng MJ, Fang F, Li M, Qin TT, Ye LY, Li HW, Qu R, Ma SP (2017) Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology 234:3385–3394. https://doi.org/10.1007/s00213-017-4720-8
Lin LT, Chung CY, Hsu WC, Chang SP, Hung TC, Shields J, Russell RS, Lin CC, Li CF, Yen MH, Tyrrell DL, Lin CC, Richardson CD (2015) Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol 62:541–548. https://doi.org/10.1016/j.jhep.2014.10.040
Lee WP, Lan KL, Liao SX, Huang YH, Hou MC, Lan KH (2019) Antiviral effect of saikosaponin B2 in combination with daclatasvir on NS5A resistance-associated substitutions of hepatitis C virus. J Chin Med Assoc 82:368–374. https://doi.org/10.1097/JCMA.0000000000000095
Omrani M, Keshavarz M, Nejad Ebrahimi S, Mehrabi M, McGaw LJ, Ali Abdalla M, Mehrbod P (2020) Potential natural products against respiratory viruses: a perspective to develop anti-COVID-19 medicines. Front Pharmacol 11:586993. https://doi.org/10.3389/fphar.2020.586993
Kim BM, Hong SH (2011) Sequential caspase-2 and caspase-8 activation is essential for saikosaponin a-induced apoptosis of human colon carcinoma cell lines. Apoptosis 16:184–197. https://doi.org/10.1007/s10495-010-0557-x
Wong VK, Zhang MM, Zhou H, Lam KY, Chan PL, Law CK, Yue PY, Liu L (2013) Saikosaponin-d enhances the anticancer potency of TNF-α via overcoming its undesirable response of activating NF-kappa B signalling in cancer cells. Evid Based Complement Alternat Med 2013:745295. https://doi.org/10.1155/2013/745295
Wang P, Ren J, Tang J, Zhang D, Li B, Li Y (2010) Estrogen-like activities of saikosaponin-d in vitro: a pilot study. Eur J Pharmacol 626:159–165. https://doi.org/10.1016/j.ejphar.2009.09.047
Chen XQ, Chen SJ, Liang WN, Wang M, Li CF, Wang SS, Dong SQ, Yi LT, Li CD (2018) Saikosaponin A attenuates perimenopausal depression-like symptoms by chronic unpredictable mild stress. Neurosci Lett 662:283–289. https://doi.org/10.1016/j.neulet.2017.09.046
Ministry of Health (2022) LaWoJ. Japanese pharmacopoeia, 18th ed. http://www.mhlw.go.jp/topics/bukyoku/iyaku/yakkyoku/english.html. Accessed 2023
Kimata H, Hiyama C, Yahara S, Tanaka O, Ishikawa O, Aiura M (1979) Application of high performance liquid chromatography to the analysis of crude drugs: Separatory determination of saponins of Bupleuri radix. Chem Pharm Bull 27:1836–1841. https://doi.org/10.1248/cpb.27.1836
Ebata N, Nakajima K, Hayashi K, Okada M, Maruno M (1996) Saponins from the root of Bupleurum falcatum. Phytochemistry 41:895–901. https://doi.org/10.1016/0031-9422(95)00720-2
Tian RT, Xie PS, Liu HP (2009) Evaluation of traditional Chinese herbal medicine: Chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J Chromatogr A 1216:2150–2155. https://doi.org/10.1016/j.chroma.2008.10.127
Li XQ, Gao QT, Chen XH, Bi KS (2005) High performance liquid chromatographic assay of saikosaponins from radix Bupleuri in China. Biol Pharm Bull 28:1736–1742. https://doi.org/10.1248/bpb.28.1736
Park IS, Kang EM, Kim N (2000) High-performance liquid chromatographic analysis of saponin compounds in Bupleurum falcatum. J Chromatogr Sci 38:229–233. https://doi.org/10.1093/chromsci/38.6.229
Lee J, Yang DH, Suh JH, Kim U, Eom HY, Kim J, Lee MY, Kim J, Han SB (2011) Species discrimination of Radix Bupleuri through the simultaneous determination of ten saikosaponins by high performance liquid chromatography with evaporative light scattering detection and electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:3887–3895. https://doi.org/10.1016/j.jchromb.2011.10.040
Eom HY, Park SY, Kim MK, Suh JH, Yeom H, Min JW, Kim U, Lee J, Youm JR, Han SB (2010) Comparison between evaporative light scattering detection and charged aerosol detection for the analysis of saikosaponins. J Chromatogr A 1217:4347–4354. https://doi.org/10.1016/j.chroma.2010.04.047
Zhou Y, Lin Y, Liu X, Ju W (2015) Simultaneous determination of 12 index components and compatibility changes in Longchai decoction by liquid chromatography-mass spectrometry. J Chromatogr Sci 53:60–65. https://doi.org/10.1093/chromsci/bmu015
Sun T, Luo J, Xu Y, Sun X, Yang S, Yang M (2021) Ultra-high performance supercritical fluid chromatography method for separation and quantitation of saikosaponins in herbal medicine. J Pharm Biomed Anal 199:114039. https://doi.org/10.1016/j.jpba.2021.114039
Ma H, Ó’Fágáin C, O’Kennedy R (2020) Antibody stability: a key to performance - analysis, influences and improvement. Biochimie 177:213–225. https://doi.org/10.1016/j.biochi.2020.08.019
Sakamoto S, Putalun W, Vimolmangkang S, Phoolcharoen W, Shoyama Y, Tanaka H, Morimoto S (2018) Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J Nat Med 72:32–42. https://doi.org/10.1007/s11418-017-1144-z
Nuntawong P, Putalun W, Tanaka H, Morimoto S, Sakamoto S (2022) Lateral flow immunoassay for small-molecules detection in phytoproducts: a review. J Nat Med 76:521–545. https://doi.org/10.1007/s11418-022-01605-6
Fujii S, Morinaga O, Uto T, Nomura S, Shoyama Y (2014) Development of a monoclonal antibody-based immunochemical assay for liquiritin and its application to the quality control of licorice products. J Agric Food Chem 62:3377–3383. https://doi.org/10.1021/jf404731z
Sakamoto S, Yusakul G, Pongkitwitoon B, Paudel MK, Tanaka H, Morimoto S (2015) Simultaneous determination of soy isoflavone glycosides, daidzin and genistin by monoclonal antibody-based highly sensitive indirect competitive enzyme-linked immunosorbent assay. Food Chem 169:127–133. https://doi.org/10.1016/j.foodchem.2014.08.004
Fujii S, Ohta T, Ehama R, Irikida M, Nomura S, Shoyama Y, Uto T (2023) Development of an indirect competitive enzyme-linked immunosorbent assay for formononetin and its application in a cell-based assay using MC3T3-E1 cells. Food Chem 403:134339. https://doi.org/10.1016/j.foodchem.2022.134339
Fujii S, Tuvshintogtokh I, Mandakh B, Munkhjargal B, Uto T, Morinaga O, Shoyama Y (2014) Screening of Glycyrrhiza uralensis Fisch. ex DC. containing high concentrations of glycyrrhizin by Eastern blotting and enzyme-linked immunosorbent assay using anti-glycyrrhizin monoclonal antibody for selective breeding of licorice. J Nat Med 68:717–722. https://doi.org/10.1007/s11418-014-0847-7
Paudel MK, Sakamoto S, Huy LV, Tanaka H, Miyamoto T, Takano A, Morimoto S (2017) Development of an immunoassay using an anti-wogonin glucuronide monoclonal antibody. J Immunoassay Immunochem 38:457–470. https://doi.org/10.1080/15321819.2016.1273236
Noguchi K, Imahori D, Kido Y, Nuntawong P, Tanaka H, Morimoto S, Sakamoto S (2023) Quality assessment method for Chinpi, dried Citrus spp. peel and its derived Kampo medicines using specific monoclonal antibody against hesperidin. Phytochem Anal 34:652–660. https://doi.org/10.1002/pca.3255
Zhu SH, Shimokawa S, Tanaka H, Shoyama Y (2004) Development of an assay system for saikosaponin a using anti-saikosaponin a monoclonal antibodies. Biol Pharm Bull 27:66–71. https://doi.org/10.1248/bpb.27.66
Sai J, Zhao Y, Shan W, Qu B, Zhang Y, Cheng J, Qu H, Wang Q (2016) Development of an enzyme-linked immunosorbent assay and immunoaffinity column chromatography for saikosaponin d using an anti-saikosaponin d monoclonal antibody. Planta Med 82:432–439. https://doi.org/10.1055/s-0035-1568182
Cui Y, Zhao J, Zhou J, Tan G, Zhao Q, Zhang Y, Wang B, Jiao B (2019) Development of a sensitive monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for analysing nobiletin in citrus and herb samples. Food Chem 293:144–150. https://doi.org/10.1016/j.foodchem.2019.04.101
Weiler EW, Zenk MH (1976) Radioimmunoassay for the determination of digoxin and related compounds in Digitalis lanata. Phytochemistry 15:1537–1545. https://doi.org/10.1016/S0031-9422(00)88933-5
Minami K, Yusakul G, Fujii S, Putalun W, Tanaka H, Sakamoto S, Morimoto S (2021) Rapid magnetic particles-based enzyme immunoassay for the quality control of Glycyrrhiza spp. based on glycyrrhizin content. Fitoterapia 148:104794. https://doi.org/10.1016/j.fitote.2020.104794
Erlanger BF (1980) The preparation of antigenic hapten-carrier conjugates: a survey. Methods Enzymol 70:85–104
Sakamoto S, Kohno T, Shimizu K, Tanaka H, Morimoto S (2016) Detection of ganoderic acid A in Ganoderma lingzhi by an indirect competitive enzyme-linked immunosorbent assay. Planta Med 82:747–751. https://doi.org/10.1055/s-0042-104202
Fujii S, Uto T, Nomura S, Shoyama Y (2018) Preparation of anti-glycyrrhetinic acid monoclonal antibody for application in an indirect competitive enzyme-linked immunosorbent assay. Anal Lett 51:1147–1162. https://doi.org/10.1080/00032719.2017.1370598
Uto T, Fujii S, Sakamoto S, Ohta T, Shoyama Y (2020) Applications of monoclonal antibodies against natural compounds for functional analysis of crude drugs. Curr Pharmacol Rep 6:192–201. https://doi.org/10.1007/s40495-020-00224-7
Funding
This study was funded by the Japan Agency for Medical Research and Development AMED (Grant No. JP21mk0101200).
Author information
Authors and Affiliations
Contributions
Seiichi Sakamoto and Hiroyuki Tanaka made substantial contributions to the conception and design of the study and supervised the research, along with Satoshi Morimoto and Takuhiro Uto. Akihiro Ochi, Shunsuke Fujii, and Tomoe Ohata conducted the experiments. Akihiro Ochi and Shunsuke Fujii conducted data analysis, wrote the first draft, and made equal contributions to this work (co-first authors). Seiichi Sakamoto and Poomraphie Nuntawong revised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ochi, A., Fujii, S., Ohta, T. et al. Highly sensitive indirect competitive enzyme-linked immunosorbent assay based on a monoclonal antibody against saikosaponin b2 for quality control of Kampo medicines containing Bupleuri radix. J Nat Med 78, 160–168 (2024). https://doi.org/10.1007/s11418-023-01753-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01753-3