Abstract
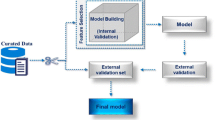
QSAR models capable of predicting biological, toxicity, and pharmacokinetic properties were widely used to search lead bioactive molecules in chemical databases. The dataset’s preparation to build these models has a strong influence on the quality of the generated models, and sampling requires that the original dataset be divided into training (for model training) and test (for statistical evaluation) sets. This sampling can be done randomly or rationally, but the rational division is superior. In this paper, we present MASSA, a Python tool that can be used to automatically sample datasets by exploring the biological, physicochemical, and structural spaces of molecules using PCA, HCA, and K-modes. The proposed algorithm is very useful when the variables used for QSAR are not available or to construct multiple QSAR models with the same training and test sets, producing models with lower variability and better values for validation metrics. These results were obtained even when the descriptors used in the QSAR/QSPR were different from those used in the separation of training and test sets, indicating that this tool can be used to build models for more than one QSAR/QSPR technique. Finally, this tool also generates useful graphical representations that can provide insights into the data.










Similar content being viewed by others
Data availability
The molecular files are available in the first author’s GitHub repository at https://github.com/gcverissimo/MASSA_datasets.
Code availability
The source code is available in the first author’s GitHub repository at https://github.com/gcverissimo/MASSA_Algorithm.
References
Yang X, Wang Y, Byrne R et al (2019) Concepts of artificial intelligence for computer-assisted drug discovery. Chem Rev 119:10520–10594. https://doi.org/10.1021/acs.chemrev.8b00728
Masand VH, Mahajan DT, Nazeruddin GM et al (2015) Effect of information leakage and method of splitting (rational and random) on external predictive ability and behavior of different statistical parameters of QSAR model. Med Chem Res 24:1241–1264. https://doi.org/10.1007/s00044-014-1193-8
Andrada MF, Vega-Hissi EG, Estrada MR, Garro Martinez JC (2017) Impact assessment of the rational selection of training and test sets on the predictive ability of QSAR models. SAR QSAR Environ Res 28:1011–1023. https://doi.org/10.1080/1062936X.2017.1397056
Clark DE (2006) What has computer-aided molecular design ever done for drug discovery? Expert Opin Drug Discov 1:103–110. https://doi.org/10.1517/17460441.1.2.103
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (2017) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk
Martin TM, Harten P, Young DM et al (2012) Does Rational selection of training and test sets improve the outcome of QSAR modeling? J Chem Inf Model 52:2570–2578. https://doi.org/10.1021/ci300338w
Cherkasov A, Muratov EN, Fourches D et al (2014) QSAR modeling: where have you been? Where are you going to? J Med Chem 57:4977–5010. https://doi.org/10.1021/jm4004285
Muratov EN, Bajorath J, Sheridan RP et al (2020) QSAR without borders. Chem Soc Rev 49:3525–3564. https://doi.org/10.1039/D0CS00098A
Puzyn T, Mostrag-Szlichtyng A, Gajewicz A et al (2011) Investigating the influence of data splitting on the predictive ability of QSAR/QSPR models. Struct Chem 22:795–804. https://doi.org/10.1007/s11224-011-9757-4
Esbensen KH, Geladi P (2010) Principles of proper validation: use and abuse of re-sampling for validation. J Chemom 24:168–187. https://doi.org/10.1002/cem.1310
Hawkins DM, Basak SC, Mills D (2003) Assessing model fit by cross-validation. J Chem Inf Comput Sci 43:579–586. https://doi.org/10.1021/ci025626i
Golbraikh A, Tropsha A (2000) Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection. Mol Divers 5:231–243. https://doi.org/10.1023/A:1021372108686
Golbraikh A, Shen M, Xiao Z et al (2003) Rational selection of training and test sets for the development of validated QSAR models. J Comput Aided Mol Des 17:241–253. https://doi.org/10.1023/A:1025386326946
Wu W, Walczak B, Massart DL et al (1996) Artificial neural networks in classification of NIR spectral data: design of the training set. Chemom Intell Lab Syst 33:35–46. https://doi.org/10.1016/0169-7439(95)00077-1
Kronenberger T, Windshügel B, Wrenger C et al (2018) On the relationship of anthranilic derivatives structure and the FXR (Farnesoid X receptor) agonist activity. J Biomol Struct Dyn 36:4378–4391. https://doi.org/10.1080/07391102.2017.1417161
Veríssimo GC, Menezes Dutra EF, Teotonio Dias AL et al (2019) HQSAR and random forest-based QSAR models for anti-T. vaginalis activities of nitroimidazoles derivatives. J Mol Graph Model 90:180–191. https://doi.org/10.1016/j.jmgm.2019.04.007
Gomes RA, Genesi GL, Maltarollo VG, Trossini GHG (2017) Quantitative structure–activity relationships (HQSAR, CoMFA, and CoMSIA) studies for COX-2 selective inhibitors. J Biomol Struct Dyn 35:1436–1445. https://doi.org/10.1080/07391102.2016.1185379
de Fernandes PO, Martins JPA, de Melo EB et al (2021) Quantitative structure-activity relationship and machine learning studies of 2-thiazolylhydrazone derivatives with anti-Cryptococcus neoformans activity. J Biomol Struct Dyn. https://doi.org/10.1080/073911021935321
Kronenberger T, Asse LR, Wrenger C et al (2017) Studies of Staphylococcus aureus FabI inhibitors: fragment-based approach based on holographic structure–activity relationship analyses. Future Med Chem 9:135–151. https://doi.org/10.4155/fmc-2016-0179
Ferreira GM, de Magalhães JG, Maltarollo VG et al (2020) QSAR studies on the human sirtuin 2 inhibition by non-covalent 7,5,2-anilinobenzamide derivatives. J Biomol Struct Dyn 38:354–363. https://doi.org/10.1080/07391102.2019.1574603
Maltarollo VG (2019) Classification of Staphylococcus aureus FabI inhibitors by machine learning techniques. IJQSPR 4:1–14. https://doi.org/10.4018/IJQSPR.2019100101
Primi MC, Maltarollo VG, Magalhães JG et al (2016) Convergent QSAR studies on a series of NK3 receptor antagonists for schizophrenia treatment. J Enzyme Inhib Med Chem 31:283–294. https://doi.org/10.3109/14756366.2015.1021250
Popova M, Isayev O, Tropsha A (2018) Deep reinforcement learning for de novo drug design. Sci Adv 4:eaap7885. https://doi.org/10.1126/sciadv.aap7885
Schneider G (2019) Mind and machine in drug design. Nat Mach Intell 1:128–130. https://doi.org/10.1038/s42256-019-0030-7
Dara S, Dhamercherla S, Jadav SS et al (2022) Machine learning in drug discovery: a review. Artif Intell Rev 55:1947–1999. https://doi.org/10.1007/s10462-021-10058-4
Ambure P, Halder AK, González Díaz H, Cordeiro MNDS (2019) QSAR-Co: an open source software for developing robust multitasking or multitarget classification-based QSAR models. J Chem Inf Model 59:2538–2544. https://doi.org/10.1021/acs.jcim.9b00295
Halder AK, Dias Soeiro Cordeiro MN (2021) QSAR-Co-X: an open source toolkit for multitarget QSAR modelling. J Cheminform 13:29. https://doi.org/10.1186/s13321-021-00508-0
Veríssimo GC (2021) MASSA Algorithm: Molecular data set sampling for training-test separation
Landrum G (2021) RDkit: 2021_03_3 (Q1 2021) Release
Vos NJ de (2015) KModes categorical clustering library
Python Software Foundation argparse—Parser for command-line options, arguments and sub-commands—Python 3.9.7 documentation. https://docs.python.org/3/library/argparse.html. Accessed 5 Oct 2021
scikit-learn: machine learning in Python—scikit-learn 1.0 documentation. https://scikit-learn.org/stable/index.html. Accessed 5 Oct 2021
sklearn.decomposition.PCA. In: scikit-learn. https://www.scikit-learn/stable/modules/generated/sklearn.decomposition.PCA.html. Accessed 5 Oct 2021
scipy.cluster.hierarchy.linkage—SciPy v1.7.1 Manual. https://docs.scipy.org/doc/scipy/reference/generated/scipy.cluster.hierarchy.linkage.html. Accessed 8 Oct 2021
scipy.cluster.hierarchy.maxdists—SciPy v1.8.0 Manual. https://docs.scipy.org/doc/scipy/reference/generated/scipy.cluster.hierarchy.maxdists.html. Accessed 22 Mar 2022
scipy.cluster.hierarchy.fcluster—SciPy v1.7.1 Manual. https://docs.scipy.org/doc/scipy/reference/generated/scipy.cluster.hierarchy.fcluster.html. Accessed 8 Oct 2021
scipy.cluster.hierarchy.dendrogram—SciPy v1.7.1 Manual. https://docs.scipy.org/doc/scipy/reference/generated/scipy.cluster.hierarchy.dendrogram.html. Accessed 8 Oct 2021
sklearn.model_selection.train_test_split. In: scikit-learn. https://www.scikit-learn/stable/modules/generated/sklearn.model_selection.train_test_split.html. Accessed 9 Oct 2021
Sutherland JJ, O’Brien LA, Weaver DF (2004) A Comparison of methods for modeling quantitative structure−activity relationships. J Med Chem 47:5541–5554. https://doi.org/10.1021/jm0497141
Liu C-J, Yu S-L, Liu Y-P et al (2016) Synthesis, cytotoxic activity evaluation and HQSAR study of novel isosteviol derivatives as potential anticancer agents. Eur J Med Chem 115:26–40. https://doi.org/10.1016/j.ejmech.2016.03.009
Valadares NF, Castilho MS, Polikarpov I, Garratt RC (2007) 2D QSAR studies on thyroid hormone receptor ligands. Bioorg Med Chem 15:4609–4617. https://doi.org/10.1016/j.bmc.2007.04.015
Ye M, Dawson MI (2009) Studies of cannabinoid-1 receptor antagonists for the treatment of obesity: hologram QSAR model for biarylpyrazolyl oxadiazole ligands. Bioorg Med Chem Lett 19:3310–3315. https://doi.org/10.1016/j.bmcl.2009.04.072
Jiao L, Wang Y, Qu L et al (2020) Hologram QSAR study on the critical micelle concentration of Gemini surfactants. Colloids Surf, A 586:124226. https://doi.org/10.1016/j.colsurfa.2019.124226
Dassault Systèmes Biovia Corp (2020) BIOVIA discovery studio visualizer 2021
Hawkins PCD, Skillman AG, Warren GL et al (2010) Conformer generation with OMEGA: algorithm and validation using high quality structures from the protein databank and Cambridge structural database. J Chem Inf Model 50:572–584. https://doi.org/10.1021/ci100031x
OMEGA 2.5.1.4. OpenEye Scientific Software, Santa Fe
QUACPAC 1.6.3.1. OpenEye Scientific Software, Santa Fe
Burns J, Spiekermann K, Bhattacharjee H, et al (2023) Machine Learning Validation via Rational Dataset Sampling with astartes
TRIPOS Associates Inc (2012) Sybyl-X Molecular Modeling Software Packages
Berthold MR, Cebron N, Dill F et al (2009) KNIME—the Konstanz information miner: version 2.0 and beyond. ACM SIGKDD Explor Newsl. https://doi.org/10.1145/16562741656280
Fernandes PO, Martins DM, de Souza BA et al (2021) Molecular insights on ABL kinase activation using tree-based machine learning models and molecular docking. Mol Divers 25:1301–1314. https://doi.org/10.1007/s11030-021-10261-z
Pedregosa F, Varoquaux G, Gramfort A et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
Virtanen P, Gommers R, Oliphant TE et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17:261–272. https://doi.org/10.1038/s41592-019-0686-2
Chirico N, Gramatica P (2011) Real external predictivity of QSAR models: how to evaluate it? comparison of different validation criteria and proposal of using the concordance correlation coefficient. J Chem Inf Model 51:2320–2335. https://doi.org/10.1021/ci200211n
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20:269–276. https://doi.org/10.1016/S1093-3263(01)00123-1
Roy K, Kar S, Das RN (2015) A primer on QSAR/QSPR modeling. Springer International Publishing, Cham
Shi LM, Fang H, Tong W et al (2001) QSAR models using a large diverse set of estrogens. J Chem Inf Comput Sci 41:186–195. https://doi.org/10.1021/ci000066d
Gramatica P, Sangion A (2016) A historical excursus on the statistical validation parameters for QSAR models: a clarification concerning metrics and terminology. J Chem Inf Model 56:1127–1131. https://doi.org/10.1021/acs.jcim.6b00088
Bae S-Y, Lee J, Jeong J et al (2021) Effective data-balancing methods for class-imbalanced genotoxicity datasets using machine learning algorithms and molecular fingerprints. Comput Toxicol 20:100178. https://doi.org/10.1016/j.comtox.2021.100178
Veríssimo GC, Serafim MSM, Kronenberger T et al (2022) Designing drugs when there is low data availability: one-shot learning and other approaches to face the issues of a long-term concern. Expert Opin Drug Discov 17:929–947. https://doi.org/10.1080/17460441.2022.2114451
Ambure P, Gajewicz-Skretna A, Cordeiro MNDS, Roy K (2019) New workflow for QSAR model development from small data sets: small dataset curator and small dataset modeler. integration of data curation, exhaustive double cross-validation, and a set of optimal model selection techniques. J Chem Inf Model 59:4070–4076. https://doi.org/10.1021/acs.jcim.9b00476
Acknowledgements
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Pró-Reitoria de Pesquisa of the Universidade Federal de Minas Gerais for financial support, OpenEye Scientific Software for OMEGA and QUACPAC academic licenses and Prof. Dr. Raquel Cardoso de Melo Minardi for her encouragement and for offering the course in which this tool was developed.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Pró-Reitoria de Pesquisa of the Universidade Federal de Minas Gerais for financial support and academic grants. OpenEye Scientific Software for OMEGA and QUACPAC academic licenses. T.K. is funded by the TüCAD2 and CMIF. TüCAD2 and CMIF are funded by the Federal Ministry of Education and Research (BMBF) and the Baden-Württemberg Ministry of Science as part of the Excellence Strategy of the German Federal and State Governments.
Author information
Authors and Affiliations
Contributions
GCV wrote the MASSA algorithm code and applied in the training test splitting, and prepared all the figures and tables. GCV and SQP generated and validated QSAR models. GCV, SQP, POF, and JCG analyzed and compared the obtained data. JCG, TK, KMH, and VGM designed the experiments, and supervised the students. All the authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests (financial or non-financial) to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Veríssimo, G.C., Pantaleão, S.Q., Fernandes, P. et al. MASSA Algorithm: an automated rational sampling of training and test subsets for QSAR modeling. J Comput Aided Mol Des 37, 735–754 (2023). https://doi.org/10.1007/s10822-023-00536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-023-00536-y