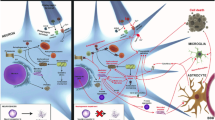
Abstract
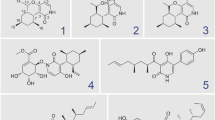
Amyotrophic lateral sclerosis (ALS) is a devastating motor disease with limited treatment options. A domestic fungal extract library was screened using three assays related to the pathophysiology of ALS with the aim of developing a novel ALS drug. 2(3H)-dihydrofuranolactones 1 and 2, and five known compounds 3–7 were isolated from Pleosporales sp. NUH322 culture media, and their protective activity against the excitotoxicity of β-N-oxalyl-L-α,β-diaminopropionic acid (ODAP), an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamatergic agonist, was evaluated under low mitochondrial glutathione levels induced by ethacrynic acid (EA) and low sulfur amino acids using our developed ODAP-EA assay. Additional assays evaluated the recovery from cytotoxicity caused by transfected SOD1-G93A, an ALS-causal gene, and the inhibitory effect against reactive oxygen species (ROS) elevation. The structures of 1 and 2 were elucidated using various spectroscopic methods. We synthesized 1 from D-ribose, and confirmed the absolute structure. Isolated and synthesized 1 displayed higher ODAP-EA activities than the extract and represented its activity. Furthermore, 1 exhibited protective activity against SOD1-G93A-induced toxicity. An ALS mouse model, SOD1-G93A, of both sexes, was treated orally with 1 at pre- and post-symptomatic stages. The latter treatment significantly extended their lifespan (p = 0.03) and delayed motor deterioration (p = 0.001–0.01). Our result suggests that 1 is a promising lead compound for the development of ALS drugs with a new spectrum of action targeting both SOD1-G93A proteopathy and excitotoxicity through its action on the AMPA-type glutamatergic receptor.
Graphical abstract










Similar content being viewed by others
References
Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA (2019) ALS genetics, mechanisms, and therapeutics: where are we now? Front Neurosci 13:1310. https://doi.org/10.3389/fnins01310
Bensimon G, Lacomblez L, Meininger V, ALS/Riluzole Study Group (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 330:585–591. https://doi.org/10.1056/NEJM199403033300901
Estevez AG, Stutzmann JM, Barbeito L (1995) Protective effect of riluzole on excitatory amino acid-mediated neurotoxicity in motoneuron-enriched cultures. Eur J Pharmacol 280:47–53. https://doi.org/10.1016/0014-2999(95)00186-o
Doble A (1996) The pharmacology and mechanism of action of riluzole. Neurology 47(6 Suppl 4):S233–S241. https://doi.org/10.1212/wnl.47.6_suppl_4.233s
Akamatsu M, Yamashita T, Hirose N, Teramoto S, Kwak S (2016) The AMPA receptor antagonist perampanel robustly rescues amyotrophic lateral sclerosis (ALS) pathology in sporadic ALS model mice. Sci Rep 6:28649. https://doi.org/10.1038/srep28649
Aoki M, Warita H, Mizuno H et al (2011) Feasibility study for functional test battery of SOD transgenic rat (H46R) and evaluation of edaravone, a free radical scavenger. Brain Res 1382:321. https://doi.org/10.1016/j.brainres.2011.01.058
Ueda T, Inden M, Shirai K, Sekine SI, Masaki Y, Kurita H, Ichihara K, Inuzuka T, Hozumi I (2017) The effects of Brazilian green propolis that contains flavonols against mutant copper-zinc superoxide dismutase-mediated toxicity. Sci Rep 7(1):2882. https://doi.org/10.1038/s41598-017-03115-y
Mancuso R, del Valle J, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Núñez O, Pallás M, Portero-Otin M, Osta R, Navarro X (2014) Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 11(2):419–432. https://doi.org/10.1007/s13311-013-0253-y
West M, Mhatre M, Ceballos A et al (2004) The arachidonic acid 5-lipoxygenase inhibitor nordihydroguairetic acid inhibits tumor necrosis activation of microglia and extends survival of G93A-SOD1 transgenic miceb. J Neurochem 91:133–143. https://doi.org/10.1111/j.1471-4159.2004.02700.x
Fontanilla CV, Wei X, Zhao L, Johnstone B, Pascuzzi RM, Farlow MR, Du Y (2011) Caffeic acid phenethyl ester extends survival of a mouse model of amyotrophic lateral sclerosis. Neuroscience 15(205):185–193. https://doi.org/10.1016/j.neuroscience.2011.12.025
Xu Z, Chen S, Li X, Luo G, Li L (2006) Le W (2006) Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res 31(10):1263–1269. https://doi.org/10.1007/s11064-006-9166-z
Dutta K, Thammisetty SS, Boutej H et al (2020) Mitigation of ALS pathology by neuron- specific inhibition of nuclear factor Kappa B signaling. J Neurosci 40:5137–5153. https://doi.org/10.1523/JNEUROSCI.0536-20
Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JP, Popovich PG, Guttridge DC, Kaspar BK (2014) Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81(5):1009–1023. https://doi.org/10.1016/j.neuron.01.013
Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301(5634):839–842. https://doi.org/10.1126/science.1086137
Azzouz M, Ralph GS, Storkebaum E et al (2004) VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429:413–417. https://doi.org/10.1038/nature02544
Bianchi VE, Locatelli V, Rizzi L (2017) Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol Sci 18(11):2441. https://doi.org/10.3390/ijms18112441
Egawa N, Kitaoka S, Tsukita K et al. (2012) Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med 4(145):145ra104. https://doi.org/10.1126/scitranslmed.3004052
Wong SQ, Pontifex MG, Phelan MM et al (2018) α-Methyl-α-phenylsuccinimide ameliorates neurodegeneration in a C. elegans model of TDP-43 proteinopathy. Neurobiol Dis 118:40–54. https://doi.org/10.1016/j.nbd.2018.06.013
Srinivasan E, Rajasekaran R (2018) Comparative binding of kaempferol and kaempferide on inhibiting the aggregate formation of mutant (G85R) SOD1 protein in familial amyotrophic lateral sclerosis: a quantum chemical and molecular mechanics study. BioFactors 4(5):431–442. https://doi.org/10.1002/biof.1441
Maurel C, Chami AA, Thépault RA, Marouillat S, Blasco H, Corcia P, Andres CR, Vourc’h PA (2020) A role for SUMOylation in the formation and cellular localization of TDP-43 aggregates in amyotrophic lateral sclerosis. Mol Neurobiol 57(3):1361–1373. https://doi.org/10.1007/s12035-019-01810-7
Hawksworth DL, Lücking R (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.FUNK-0018-2016
Willis CL, Meldrum BS, Nunn PB, Anderton BH, Leigh PN (1993) Neuronal damage induced by beta-N-oxalylamino-L-alanine, in the rat hippocampus, can be prevented by a non-NMDA antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline. Brain Res 627(1):55–62. https://doi.org/10.1016/0006-8993(93)90748-c.
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698(1–3):6–18. https://doi.org/10.1016/j.ejphar.2012.10.032
Kusama-Eguchi K, Miyano T, Yamamoto M et al (2014) New insight into the mechanism of neurolathyrism: L-ß-ODAP triggers [Ca2+]i accumulation and cell death in primary motor neurons through transient receptor potential channels and metabotropic glutamate receptors. Food Chem Toxicol 67:113–22. https://doi.org/10.1016/j.fct.2014.02.021
Cashman NR, Durham HD, Blusztajn JK et al (1992) Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn 194:209–221. https://doi.org/10.1002/aja.1001940306
Eggett CJ, Crosier S, Manning P et al (2000) Development and characterization of a glutamate-sensitive motor neuron cell line. J Neurochem 74:1895–1902. https://doi.org/10.1046/j.1471-4159.2000.0741895.x
Ravindranath V (2002) Neurolathyrism: mitochondrial dysfunction in excitotoxicity mediated by L-beta-oxalyl aminoalanine. Neurochem Int 40(6):505–509. https://doi.org/10.1016/s0197-0186(01)00121-8
Kusama-Eguchi K, Yoshino N, Minoura A et al (2011) Sulfur amino acids deficiency cause by grass pea diet plays an important role in the toxicity of L-ß-ODAP by increasing the oxidative stress: Studies on a motor neuron cell line. Food Chem Toxicol 49:636–643. https://doi.org/10.1016/j.fct.2010.07.049
Dukhande VV, Kawikova I (2013) Neuroprotection against neuroblastoma cell death induced by depletion of mitochondrial glutathione. Apoptosis 18:702. https://doi.org/10.1007/s10495-013-0836-4
Trumbull KA, McAllister D, Gandelman MM, Fung WY, Lew T, Brennan L, Lopez N, Morré J, Kalyanaraman B, Beckman JS (2012) Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol 45(1):137–144. https://doi.org/10.1016/j.nbd.2011.07.015
Urushitani M, Kurisu J, Tsukita K, Takahashi R (2002) Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. J Neurochem 83:1030–1042. https://doi.org/10.1046/j.1471-4159.2002.01211.x
Hyun DH, Lee M, Halliwell B, Jenner P (2003) Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J Neurochem 86(2):363–373. https://doi.org/10.1046/j.1471-4159.2003.01841.x
Hirose D, Shirouzu T, Hirota M, Ohtsuka T, Senga T, Du M, Shimono A, Zhang X (2009) Species richness and species composition of fungal communities associated with cellulose decomposition at different altitudes on the Tibetan Plateau. J Plant Ecolo 2:217–224. https://doi.org/10.1093/jpe/rtp028
Evidente A, Sparapano L, Fierro O, Bruno G, Motta A (1999) Sapinofuranones A and B, two new 2(3H)-dihydrofuranones produced by sphaeropsis sapinea, a common pathogen of conifers. J Nat Prod 62(2):253–256. https://doi.org/10.1021/np980318t
Mondol MA, Farthouse J, Islam MT, Schüffler A, Laatsch H (2017) Metabolites from the endophytic fungus Curvularia sp. M12 Act as motoity inhibitors against Phytophthora capsici zoospores. J Nat Prod 80(2):347–355. https://doi.org/10.1021/acs.jnatprod.6b00785
Masi M, Maddau L, Linaldeddu BT, Cimmino A, D'Amico W, Scanu B, Evidente M, Tuzi A, Evidente A (2016) Bioactive secondary metabolites produced by the oak pathogen Diplodia corticola. J Agric Food Chem 64: 217–225. https://doi.org/10.1021/acs.jafc.5b05170
Suciati FJA, Lambert LK, Pierens GK, Bernhardt PV, Garson MJ (2013) Secondary metabolites of the sponge-derived fungus Acremonium persicinum. J Nat Prod 76(8):1432–1440. https://doi.org/10.1021/np4002114
Nagarapu L, Karnakanti S, Bantu S (2012) Total synthesis of sapinofuranone A from D-ribose. Tetrahedron 68:5829–5832. https://doi.org/10.1026/j.tet.2012.05.012
Yadav JS, Mandal SS, Reddy JSS, Srihari P (2011) Stereoselective total synthesis of (+)-sapinofuranone B. Tetrahedron 67:4620–4627. https://doi.org/10.1016/j.tet.2011.04.072
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database System Rev. https://doi.org/10.1002/14651858.CD001447.pub3
Writing Group (2017) Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 16:505–512. https://doi.org/10.1016/S1474-4422(17)30115-1
Okano H, Yasuda D, Fujimori K, Morimoto S, Takahashi S (2020) Ropinirole, a new ALS drug candidate developed using iPSCs. Trends Pharmacol Sci 41(2):99–109. https://doi.org/10.1016/j.tips.2019.12.002
Osaki T, Uzel SGM, Kamm RD (2018) Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci Adv 10;4(10):eaat5847. https://doi.org/10.1126/sciadv.aat5847
Miller TM, Cudkowicz ME, Genge A, Shaw PJ, Sobue G et al (2022) Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N Engl J Med 387(12):1099–1110. https://doi.org/10.1056/NEJMoa2204705
Handley EE, Reale LA, Chuckowree JA, Dyer MS, Barnett GL, Clark CM, Bennett W, Dickson TC, Blizzard CA (2022) Estrogen enhances dendrite spine function and recovers deficits in neuroplasticity in the prpTDP-43A315T mouse model of amyotrophic lateral sclerosis. Mol Neurobiol 59(5):2962–2976. https://doi.org/10.1007/s12035-022-02742-535249200
Van Damme P, Leyssen M, Callewaert G, Robberecht W, Van Den Bosch L (2003) The AMPA receptor antagonist NBQX prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett 343:81–84. https://doi.org/10.1016/s0304-3940(03)00314-8
Heath PR, Shaw PJ (2002) Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve 26(4):438–58. https://doi.org/10.1002/mus.10186
Dong L, Kim HJ, Cao TQ, Liu Z, Lee H, Ko W, Kim YC, Sohn JH, Kim TK, Yim JH, Lee DS, Oh H (2021) Anti-inflammatory effects of metabolites from antarctic fungal strain Pleosporales sp. SF-7343 in HaCaT human keratinocytes. Int J Mol Sci 22(18):9674. https://doi.org/10.3390/ijms22189674
Liu J, Lillo C, Jonsson PA et al (2004) Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron 43:5–17. https://doi.org/10.1016/j.neuron.2004.06.016
Vijayvergiya C, Beal MF, Buck J, Manfredi G (2005) Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci 25(10):2463–2470. https://doi.org/10.1523/JNEUROSCI.4385-04.2005
ADF2019, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, http://www.scm.com
Barber SC, Higginbottom A, Mead RJ, Barber S, Shaw PJ (2009) An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Radic Biol Med. 46(8):1127–1138. https://doi.org/10.1016/j.freeradbiomed.2009.01.019
Acknowledgements
We are grateful to Mr. Masaki Watanabe, M.S., and Mr. Atsuhiro Suda, B.S., for their excellent assistance in obtaining and analyzing some of the in vitro data.
Funding
Part of this work was supported by A Grant from the Ministry of Education, Culture, Sports, Science, and Technology to promote 2001-multidisciplinary research projects (2007–2011), Nihon University Multidisciplinary Research Grant No. 12-021 and 13-023 (2012–2013), and Grant-in-Aid for Scientific Research (Kuniko Kusama No. 22590088 (2010–2012).
Author information
Authors and Affiliations
Contributions
KKE, YT, AM, YY, and KM contributed to collecting data in the screening of fungal extracts and other in vitro assays. KKE, YY, and YK contributed and analyzed the animal experiment data. DH and YO have established the fungal extracts library and identified NUH322 and other fungi listed in this report. YT, YY, MF, EO, MM, KM, and AO contributed to obtaining and analyzing the natural substances from Pleosporales sp. NUH322. MM contributed to the synthesis of compound 1. KW supervised the whole biological experiments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kusama-Eguchi, K., Tokui, Y., Minoura, A. et al. 2(3H)-Dihydrofranolactone metabolites from Pleosporales sp. NUH322 as anti-amyotrophic lateral sclerosis drugs. J Nat Med 78, 146–159 (2024). https://doi.org/10.1007/s11418-023-01751-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01751-5