Abstract
Background
Acetaminophen (APAP) overdose can cause severe acute liver injury. Poly (ADP-ribose) polymerase 1 (PARP1) is a nuclear protease that senses DNA breaks and repairs damaged DNA. The role PARP1 plays in APAP-induced hepatotoxicity is still unclear.
Materials and methods
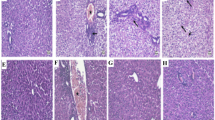
The study was designed in two parts. First, the relationship between PARP1 expression and hepatotoxicity was investigated. Then, the inhibitor PJ34 was used to inhibit the activity of PARP1 and examined its effects. In particular, APAP, vehicle or PJ34 was intraperitoneally administered to mice. Serum transaminase levels were measured with commercial kits. Hematoxylin & eosin staining was used for histopathological observation of the liver. The protein levels of PARP1, poly (ADP-ribose), Sirtuin1 (Sirt1) and γ-H2AX were detected by western blotting.
Results
In a dose- and time-dependent manner, APAP exposure resulted in the overactivation of PARP1 in the livers of mice. Posttreatment with PJ34 ameliorated changes in serum transaminase levels, and histopathological abnormalities. The protein expression of Sirt1 was elevated by PJ34, while that of PARP1, poly (ADP-ribose), and γ-H2AX was reduced due to PJ34 administration.
Conclusion
Excessive APAP administration results in PARP1 overactivation, and its inhibition sheds light on the treatment of APAP-induced liver injury.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Cerutti R et al (2014) NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 19(6):1042–1049
Chen D et al (2019) p53 Up-regulated modulator of apoptosis induction mediates acetaminophen-induced necrosis and liver injury in mice. Hepatology 69(5):2164–2179
Chen Y et al (2020) c-Jun NH(2) -terminal protein kinase phosphorylates the Nrf2-ECH homology 6 domain of nuclear factor erythroid 2-related factor 2 and downregulates cytoprotective genes in acetaminophen-induced liver injury in mice. Hepatology 71(5):1787–1801
Chowdhury A et al (2019) Mangiferin ameliorates acetaminophen-induced hepatotoxicity through APAP-Cys and JNK modulation. Biomed Pharmacother 117:109097
Cover C et al (2005) Pathophysiological role of poly(ADP-ribose) polymerase (PARP) activation during acetaminophen-induced liver cell necrosis in mice. Toxicol Sci 84(1):201–208
Curtin N, Szabo C (2020) Poly(ADP-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov 19(10):711–736
Dönmez M et al (2015) PARP inhibition prevents acetaminophen-induced liver injury and increases survival rate in rats. Turk J Med Sci 45(1):18–26
Fernandez-Checa JC et al (2021) Advanced preclinical models for evaluation of drug-induced liver injury—consensus statement by the European Drug-Induced Liver Injury Network [PRO-EURO-DILI-NET]. J Hepatol 75(4):935–959
Huo Y et al (2017) Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid Redox Signal 26(5):207–220
Jagtap P et al (2002) Novel phenanthridinone inhibitors of poly (adenosine 5’-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit Care Med 30(5):1071–1082
Jang K, Hwang Y, Kim E (2020) PARP1 impedes SIRT1-mediated autophagy during degeneration of the retinal pigment epithelium under oxidative stress. Mol Cells 43(7):632–644
Lee D et al (2020) Peroxiredoxin 6 mediates acetaminophen-induced hepatocyte death through JNK activation. Redox Biol 32:101496
Lee Y et al (2014) Poly (ADP-ribose) in the pathogenesis of Parkinson’s disease. BMB Rep 47(8):424–432
Liao C et al (2022) Nicotinamide adenine dinucleotide attenuates acetaminophen-induced acute liver injury via activation of PARP1, Sirt1, and Nrf2 in mice. Can J Physiol Pharmacol 100(8):796–805
Louvet A et al (2021) Acute liver injury with therapeutic doses of acetaminophen: a prospective study. Hepatology 73(5):1945–1955
Mukhopadhyay P et al (2017) PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J Hepatol 66(3):589–600
Song X et al (2016) Unpredicted downregulation of RAD51 suggests genome instability induced by tetrachlorobenzoquinone. Chem Res Toxicol 29(12):2184–2193
Song Z et al (2008) Inhibition of the activity of poly (ADP-ribose) polymerase reduces heart ischaemia/reperfusion injury via suppressing JNK-mediated AIF translocation. J Cell Mol Med 12(4):1220–1228
Sun Y et al (2018) Liver-specific deficiency of unc-51 like kinase 1 and 2 protects mice from acetaminophen-induced liver injury. Hepatology 67(6):2397–2413
Tak J et al (2022) Galpha(12) overexpression in hepatocytes by ER stress exacerbates acute liver injury via ROCK1-mediated miR-15a and ALOX12 dysregulation. Theranostics 12(4):1570–1588
Tufan AB et al (2022) TSG101 associates with PARP1 and is essential for PARylation and DNA damage-induced NF-kappaB activation. EMBO J 41(12):e110372
Wang C et al (2018) Poly(ADP-ribosyl)ated PXR is a critical regulator of acetaminophen-induced hepatotoxicity. Cell Death Dis 9(8):819
Wang L et al (2020) Genistein protects against acetaminophen-induced liver toxicity through augmentation of SIRT1 with induction of Nrf2 signalling. Biochem Biophys Res Commun 527(1):90–97
Zha S et al (2018) PARP1 inhibitor (PJ34) improves the function of aging-induced endothelial progenitor cells by preserving intracellular NAD levels and increasing SIRT1 activity. Stem Cell Res Ther 9(1):224
Zhang L, Li D (2019) MORC2 regulates DNA damage response through a PARP1-dependent pathway. Nucleic Acids Res 47(16):8502–8520
Zheng L et al (2017) JNK activation contributes to oxidative stress-induced parthanatos in glioma cells via increase of intracellular ROS production. Mol Neurobiol 54(5):3492–3505
Zhong X et al (2021) Hepatic NF-kappaB-inducing kinase and inhibitor of NF-kappaB kinase subunit alpha promote liver oxidative stress, ferroptosis, and liver injury. Hepatol Commun 5(10):1704–1720
Acknowledgements
The authors thank the Chongqing Yike Tianya Biotechnology Co., Ltd for the assistance in H&E and PAS staining.
Funding
This work was supported by the program for Youth Innovation in Future Medicine from Chongqing Medical University (Grant No. W0057).
Author information
Authors and Affiliations
Contributions
LL designed the research study. JT and CL conducted the experiments, acquired data and wrote the manuscript. KH, LL and YY revised the manuscript and corrected the figure results. JH and LT analyzed the data. LZ provided reagents. All the authors participated in manuscript preparation and approved the final manuscript. JT and CL contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interest
Jiarui Tang declares that she has no conflicts of interest. Cuiting Liao declares that she has no conflicts of interest. Kai Hu declares that he has no conflicts of interest. Longhui Li declares that she has no conflicts of interest. Yongqiang Yang declares that he has no conflicts of interest. Jiayi Huang declares that she has no conflicts of interest. Li Tang declares that she has no conflicts of interest. Li Zhang declares that he has no conflicts of interest. Longjiang Li declares that he has no conflicts of interest.
Ethics approval
The Chongqing Medical University Committee approved all experimental procedures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, J., Liao, C., Hu, K. et al. Inhibiting poly (ADP-ribose) polymerase 1 activation alleviates acetaminophen-induced acute liver injury in mice. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00400-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00400-y