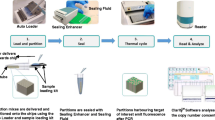
Abstract—Digital PCR (dPCR) is a nucleic acid quantification method that is widely used in genetic analysis. One of the most significant advantages of dPCR over other methods is the possibility of absolute quantitative determination of genetic material without construction of calibration curves, which allows one to detect even single molecules of nucleic acids, and, hence, provides early diagnosis of diseases. One specific characteristic of dPCR is the detection of the analyzed biological object in each microreaction, followed by the presentation of the analysis results in a binary system, thereby giving the method its name. The key aspects of developing the dPCR method, i.e., from the first devices based on microfluidic chip technology to modern systems capable of measuring a target at a concentration of up to 1 in 100 000 copies are shown in the current work. We analyzed the data on the detection of various pathogens using dPCR, as well as summarizing various study results demonstrating the innovativeness of this method. Both the possibilities of multiplex dPCR analysis and its potential in clinical practice are presented. This review also addresses the issue of the role of dPCR in the development of noninvasive methods for analysis of oncological diseases. Possible ways of developing dPCR technology were emphasized, including its use as a “point-of-care” system.
Similar content being viewed by others
REFERENCES
Lei S. 2021. Digital PCR for accurate quantification of pathogens: Principles, applications, challenges and future prospects. Int. J. Biol. Macromol. 10, 750‒759.
Vynck M., Trypsteen W., Thas O., Vandekerckhove L., De Spiegelaere W. 2016. The future of digital polymerase chain reaction in virology. Mol. Diagn. Ther. 20, 437–447.
Huggett J.F., Cowen S., Foy C.A. 2015. Considerations for digital PCR as an accurate molecular diagnostic tool. Clin. Chem. 61, 79–88.
Zhu X., Liu P., Lu L., Zhong H., Xu M., Jia R., Su L., Cao L., Sun Y., Guo M., Sun J., Xu J. 2022. Development of a multiplex droplet digital PCR assay for detection of enterovirus, parechovirus, herpes simplex virus 1 and 2 simultaneously for diagnosis of viral CNS infections. Virol. J. 19, 70.
Leong N.K.C., Chu D.K.W., Chu J.T.S., Tam Y.H., Ip D.K.M., Cowling B.J., Poon L.L.M. 2020. A six-plex droplet digital RT-PCR assay for seasonal influenza virus typing, subtyping, and lineage determination. Influenza Other Respir. Viruses. 14, 720–729.
Whale A.S., Huggett J.F., Tzonev S. 2016. Fundamentals of multiplexing with digital PCR. Biomol. Detect. Quantif. 10, 15–23.
The dMIQE Group, Huggett J.F. 2020. The digital MIQE guidelines update: Minimum information for publication of quantitative digital PCR experiments for 2020. Clin. Chem. 66, 1012–1029.
Mao X., Liu C., Tong H., Chen Y., Liu K. 2019. Principles of digital PCR and its applications in current obstetrical and gynecological diseases. Am. J. Transl. Res. 11, 7209–7222.
Chen B. 2021. Droplet digital PCR as an emerging tool in detecting pathogens nucleic acids in infectious diseases. Clin. Chim. Acta. 517, 156‒161.
Basu A.S. 2017. Digital assays part I: Partitioning statistics and digital PCR. SLAS Technol. 22, 369–386.
Pinheiro L.B., Coleman V.A., Hindson C.M., Herrmann J., Hindson B.J., Bhat S., Emslie K.R. 2012. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal. Chem. 84, 1003–1011.
Wang K., Li B., Guo Y., Wu Y., Li Y., Wu W. 2022. An integrated digital PCR system with high universality and low cost for nucleic acid detection. Front. Bioeng. Biotechnol. 10, 947895.
Hall Sedlak R., Jerome K.R. 2014. The potential advantages of digital PCR for clinical virology diagnostics. Expert. Rev. Mol. Diagn. 14, 501–507.
Tang L., Sun Y., Buelow D., Gu Z., Caliendo A.M., Pounds S., Hayden R.T. 2016. Quantitative assessment of commutability for clinical viral load testing using a digital PCR-based reference standard. J. Clin. Microbiol. 54, 1616–1623.
Sedlak R.H., Nguyen T., Palileo I., Jerome K.R., Kuypers J. 2017. Superiority of digital reverse transcription-PCR (RT-PCR) over real-time RT-PCR for quantitation of highly divergent human rhinoviruses. J. Clin. Microbiol. 55, 442–449.
Sedlak R.H., Jerome K.R. 2013. Viral diagnostics in the era of digital polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 75, 1–4.
Hudecova I. 2015. Digital PCR analysis of circulating nucleic acids. Clin. Biochem. 48, 948–956.
Sanders R., Mason D.J., Foy C.A., Huggett J.F. 2013. Evaluation of digital PCR for absolute RNA quantification. Anal. Chem. 8, e75296.
Hayden R.T., Gu Z., Sam S.S., Sun Y., Tang L., Pounds S., Caliendo A.M. 2016. Comparative performance of reagents and platforms for quantitation of c-ytomegalovirus DNA by digital PCR. J. Clin. Microbiol. 54, 2602–2608.
Vogelstein B., Kinzler K.W. 1999. Digital PCR. Proc. Natl. Acad. Sci. U. S. A. 96, 9236–9241.
Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Higuchi R., Horn G.T., Mullis K.B., Erlich H.A. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 239, 487–491.
Morley A.A. 2014. Digital PCR: A brief history. Biomol. Detect. Quantif. 1, 1–2.
Simmonds P., Balfe P., Peutherer J.F., Ludlam C.A., Bishop J.O., Brown A.J. 1990. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J. Virol. 64, 864–872.
Sykes P.J., Neoh S.H., Brisco M.J., Hughes E., Condon J., Morley A.A. 1992. Quantitation of targets for PCR by use of limiting dilution. BioTechniques. 13, 444–449.
Sanders R., Huggett J.F., Bushell C.A., Cowen S., Scott D.J., Foy C.A. 2011. Evaluation of digital PCR for absolute DNA quantification. Anal. Chem. 83, 6474–6484.
Qin J., Jones R.C., Ramakrishnan R. 2008. Studying copy number variations using a nanofluidic platform. Nucleic Acids Res. 36, e116.
Pekin D., Skhiri Y., Baret J.-C., Corre D.L., Mazutis L., Salem C.B., Millot F., Harrak A.E., Hutchison J.B., Larson J.W., Link D.R., Laurent-Puig P., Griffiths A.D., Taly V. 2011. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab. Chip. 11, 2156–2166.
Tan L.L., Loganathan N., Agarwalla S., Yang C., Yuan W., Zeng J., Wu R., Wang W., Duraiswamy S. 2022. Current commercial dPCR platforms: Technology and market review. Crit. Rev. Biotechnol. 1–32.
Garzarelli V., Chiriacò M.S., Cereda M., Autuori I., Ferrara F. 2022. Miniaturized real-time PCR systems for SARS-CoV-2 detection at the Point-of-Care. Clin. Chim. Acta. 536, 104–111.
Cassinari K., Alessandri-Gradt E., Chambon P., Charbonnier F., Gracias S., Beaussire L., Alexandre K., Sarafan-Vasseur N., Houdayer C., Etienne M., Caron F., Plantier J.C., Frebourg T. 2021. Assessment of multiplex digital droplet RT-PCR as a diagnostic tool for SARS-CoV-2 detection in nasopharyngeal swabs and saliva samples. Clin. Chem. 67, 736–741.
Artika I.M., Wiyatno A., Ma’roef C.N. 2020. Pathogenic viruses: Molecular detection and characterization. Infect. Genet. Evol. 81, 104215.
Brunetto G.S., Massoud R., Leibovitch E.C., Caruso B., Johnson K., Ohayon J., Fenton K., Cortese I., Jacobson S. 2014. Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations. J. Neurovirol. 20, 341–351.
White R.A., Quake S.R., Curr K. 2012. Digital PCR provides absolute quantitation of viral load for an occult RNA virus. J. Virol. Methods. 179, 45–50.
Wouters Y., Dalloyaux D., Christenhusz A., Roelofs H.M.J., Wertheim H.F., Bleeker-Rovers C.P., te Morsche R.H., Wanten G.J.A. 2020. Droplet digital polymerase chain reaction for rapid broad-spectrum detection of bloodstream infections. Microb. Biotechnol. 13, 657–668.
Mancusi A., Fulgione A., Girardi S., Di Maro O., Capuano F., Proroga Y.T.R., Cristiano D. 2022. Droplet digital PCR (ddPCR) analysis for detecting shiga-toxin-producing Escherichia coli (STEC). Appl. Sci. 12, 3654.
Shao Z., Zhu J., Wei Y., Jin J., Zheng Y., Liu J., Zhang R., Sun R., Hu B. 2022. Pathogen load and species monitored by droplet digital PCR in patients with bloodstream infections: A prospective case series study. BMC Infect. Dis. 22, 771.
Merino I., de la Fuente A., Domínguez-Gil M., Eiros J.M., Tedim A.P., Bermejo-Martín J.F. 2022. Digital PCR applications for the diagnosis and management of infection in critical care medicine. Crit. Care. 26, 63.
Hu B., Tao Y., Shao Z., Zheng Y., Zhang R., Yang X., Liu J., Li X., Sun R. 2021. A comparison of blood pathogen detection among droplet digital PCR, metagenomic next-generation sequencing, and blood culture in critically ill patients with suspected bloodstream infections. Front. Microbiol. 12, 641202.
Kondrashin A.V., Morozova L.F., Stepanova E.V., Turbabina N.A., Maksimova M.S., Morozov E.N. 2018. On the epidemiology of Plasmodium vivax malaria: Past and present with special reference to the former USSR. Malar. J. 17, 346.
Mangold K.A., Manson R.U., Koay E.S.C., Stephens L., Regner M., Thomson R.B., Peterson L.R., Kaul K.L. 2005. Real-time PCR for detection and identification of Plasmodium spp. J. Clin. Microbiol. 43, 2435–2440.
Koepfli C., Nguitragool W., Hofmann N.E., Robinson L.J., Ome-Kaius M., Sattabongkot J., Felger I., Mueller I. 2016. Sensitive and accurate quantification of human malaria parasites using droplet digital PCR (ddPCR). Sci. Rep. 6, 39183.
Gentilini F., Turba M.E., Taddei F., Gritti T., Fantini M., Dirani G., Sambri V. 2021. Modelling RT-qPCR cycle-threshold using digital PCR data for implementing SARS-CoV-2 viral load studies. PLoS One. 16, e0260884.
Li Y., Yao L., Li J., Chen L., Song Y., Cai Z., Yang C. 2020. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 92, 903–908.
Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D., Zhang C., Yu J., Jiang B., Cao H., Li L. 2019. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: A multicenter descriptive study. Clin. Infect. Dis. 71, 706–712.
Tahamtan A., Ardebili A. 2020. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert. Rev. Mol. Diagn. 20, 453–454.
Konka A., Lejawa M., Gaździcka J., Bochenek A., Fronczek M., Strzelczyk J.K. 2022. RT-PCR detection of SARS-CoV-2 among individuals from the upper Silesian region—analysis of 108516 tests. Diagnostics. 12, 7.
Suo T., Liu X., Feng J., Guo M., Hu W., Guo D., Ullah H., Yang Y., Zhang Q., Wang X., Sajid M., Huang Z., Deng L., Chen T., Liu F., Xu K., Liu Y., Zhang Q., Liu Y., Xiong Y., Chen G., Lan K., Chen Y. 2020. ddPCR: A more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg. Microbes Infect. 9, 1259–1268.
Dong L., Zhou J., Niu C., Wang Q., Pan Y., Sheng S., Wang X., Zhang Y., Yang J., Liu M., Zhao Y., Zhang X., Zhu T., Peng T., Xie J., Gao Y., Wang D., Dai X., Fang X. 2021. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 224, 121726.
Telysheva E.N. 2017. Free-circulating plasma DNA: Potential applications in oncology. Vestn. RNTsRR. 17, 2.
Sarhadi V.K., Armengol G. 2022. Molecular biomarkers in cancer. Biomolecules. 12, 1021.
Wang S., Zhang K., Tan S., Xin J., Yuan Q., Xu H., Xu X., Liang Q., Christiani D.C., Wang M., Liu L., Du M. 2021. Circular RNAs in body fluids as cancer biomarkers: The new frontier of liquid biopsies. Mol. Cancer. 20, 13.
Szilágyi M., Pös O., Márton É., Buglyó G., Soltész B., Keserű J., Penyige A., Szemes T., Nagy B. 2020. Circulating cell-free nucleic acids: Main characteristics and clinical application. Int. J. Mol. Sci. 21, 6827.
Pös O., Biró O., Szemes T., Nagy B. 2018. Circulating cell-free nucleic acids: Characteristics and applications. Eur. J. Hum. Genet. 26, 937–945.
Liu D., Yin H., Wang Y., Cao Y., Yin J., Zhang J., Yin H., Zhao X. 2021. Development of a highly sensitive digital PCR assay to quantify long non-coding RNA MYU in urine samples which exhibited great potential as an alternative diagnostic biomarker for prostate cancer. Transl. Androl. Urol. 10, 3815–3825.
Du M., Huang C.-C., Tan W., Kohli M., Wang L. 2020. Multiplex digital PCR to detect amplifications of specific androgen receptor loci in cell-free DNA for prognosis of metastatic castration-resistant prostate cancer. Cancers. 12, E2139.
Bit-Sava E.M. 2014. Hereditary characteristics of BRCA1 of 5382insC/hek2/blm-associated breast cancer. Sib. Onkol. Zh. 6, 15–18.
Mehta A., Diwan H., Gupta G., Nathany S., Agnihotri S., Dhanda S. 2022. Founder BRCA1 mutations in Nepalese population. J. Pathol. Transl. Med. 56, 212–216.
Tsyganov M.M., Tarabanovskaya N.A., Deryusheva I.V., Ibragimova M.I., Kazantseva P.V., Pevzner A.M., Slonimskaya E.M., Litvyakov N.V. 2019. Response to neoadjuvant chemotherapy with inclusion of platinum drugs in a breast cancer patient with BRCA1 gene deletion in the tumor. Sib. Onkol. Zh. 18, 103–108.
Kayukova E.V. 2019. Possibilities of liquid biopsy in the diagnosis and monitoring of cervical cancer. Sib. Onkol. Zh. 18, 92–101.
Tewari K.S., Sill M.W., Monk B.J., Penson R.T., Moore D.H., Lankes H.A., Ramondetta L.M., Landrum L.M., Randall L.M., Oaknin A., Leitao M.M., Eisenhauer E.L., DiSilvestro P., Van Le L., Pearl M.L., Burke J.J., Salani R., Richardson D.L., Michael H.E., Kindelberger D.W., Birrer M.J. 2020. Circulating tumor cells in advanced cervical cancer: NRG oncology-gynecologic oncology group study 240 (NCT 00803062). Mol. Cancer Ther. 19, 2363–2370.
Buleje J., Guevara-Fujita M., Acosta O., Huaman F.D.P., Danos P., Murillo A., Pinto J.A., Araujo J.M., Aguilar A., Ponce J., Vigil C., Castaneda C., Calderon G., Gomez H.L., Fujita R. 2017. Mutational analysis of BRCA1 and BRCA2 genes in Peruvian families with hereditary breast and ovarian cancer. Mol. Genet. Genomic Med. 5, 481–494.
Weigelt B., Comino-Méndez I., de Bruijn I., Tian L., Meisel J.L., García-Murillas I., Fribbens C., Cutts R., Martelotto L.G., Ng C.K., Lim R.S., Selenica P., Piscuoglio S., Aghajanian C., Norton L., Murali R., Hyman D.M., Borsu L., Arcila M.E., Konner J., Reis-Filho J.S., Greenberg R.A., Robson M.E., Turner N.C. 2017. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin. Cancer Res. 23, 6708–6720.
He H.-J., Almeida J.L., Lund S.P., Steffen C.R., Choquette S., Cole K.D. 2016. Development of NIST standard reference material 2373: Genomic DNA standards for HER2 measurements. Biomol. Detect. Quantif. 8, 1–8.
Zhou R., Cai Y., Shen S., Sha M., Li Z., Head S.R., Wang Y. 2018. A digital PCR based assay to detect all ALK fusion species. Front. Lab. Med. 2, 49–54.
Beck J., Bierau S., Balzer S., Andag R., Kanzow P., Schmitz J., Gaedcke J., Moerer O., Slotta J.E., Walson P., Kollmar O., Oellerich M., Schütz E. 2013. Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin. Chem. 59, 1732–1741.
Mair R., Mouliere F. 2022. Cell-free DNA technologies for the analysis of brain cancer. Br. J. Cancer. 126, 371–378.
McEwen A.E., Leary S.E.S., Lockwood C.M. 2020. Beyond the blood: CSF-derived cfDNA for diagnosis and characterization of CNS tumors. Front. Cell Dev. Biol. 8, 45.
Bouchè V., Aldegheri G., Donofrio C.A., Fioravanti A., Roberts-Thomson S., Fox S.B., Schettini F., Generali D. 2021. BRAF signaling inhibition in glioblastoma: Which clinical perspectives? Front. Oncol. 11, 772052.
Castel D., Philippe C., Calmon R., Le Dret L., Truffaux N., Boddaert N., Pagès M., Taylor K.R., Saulnier P., Lacroix L., Mackay A., Jones C., Sainte-Rose C., Blauwblomme T., Andreiuolo F., Puget S., Grill J., Varlet P., Debily M.-A. 2015. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. (Berl.). 130, 815–827.
Zaytseva M., Usman N., Salnikova E., Sanakoeva A., Valiakhmetova A., Chervova A., Papusha L., Novichkova G., Druy A. 2022. Methodological challenges of digital PCR detection of the histone H3 K27M somatic variant in cerebrospinal fluid. Pathol. Oncol. Res. 28, 1610024.
Sahu R., Vishnuraj M.R., Srinivas Ch., Dadimi B., Megha G.K., Pollumahanti N., Malik S.S., Vaithiyanathan S., Rawool D.B., Barbuddhe S.B. 2021. Development and comparative evaluation of droplet digital PCR and quantitative PCR for the detection and quantification of Chlamydia psittaci. J. Microbiol. Methods. 190, 106318.
ACKNOWLEDGMENTS
The authors express their sincere gratitude to the Krasnoyarsk Regional Center of Research Equipment of Federal Research Center Krasnoyarsk Science Center Siberian Branch, Russian Academy of Sciences for providing the digital PCR QIAcuity One 5plex Instrument (QIAGEN, 2021) for use.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on the welfare of animals. This article does not contain any studies using animals as subjects.
Conflict of interest. The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kopylova, K.V., Kasparov, E.W., Marchenko, I.V. et al. Digital PCR as a Highly Sensitive Diagnostic Tool: A Review. Mol Biol 57, 793–801 (2023). https://doi.org/10.1134/S0026893323050059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323050059