Abstract
As part of a long-term study on the effects of nitrogen (N) loading in a shallow temperate lagoon, we measured rates of N2 fixation associated with seagrass (Zostera marina) epiphytes during the summer from 2005 to 2019, at two sites along a gradient from where high N groundwater enters the system (denoted SH) to a more well-flushed outer harbor (OH). The data presented here are the first such long-term N2 fixation estimates for any seagrass system and one of the very few reported for the phyllosphere in a temperate system. Mean daily N2 fixation was estimated from light and dark measurements using the acetylene reduction assay intercalibrated using both incorporation of 15N2 into biomass and a novel application of the N2:Ar method. Surprisingly, despite a large inorganic N input from a N-contaminated groundwater plume, epiphytic N2 fixation rates were moderately to very high for a seagrass system (OH site 14-year mean of 0.94 mmol N m−2 d−1), with the highest rates (2.6 mmol N m−2 d−1) measured at the more N-loaded eutrophic site (SH) where dissolved inorganic N was higher and soluble reactive phosphorus was lower than in the better-flushed OH. Over 95% of the total N2 fixation measured was in the light, suggesting the importance of cyanobacteria in the epiphyte assemblages. We observed large inter-annual variation both within and across the two study sites (range from 0.1 to 2.6 mmol N fixed m−2 d−1), which we suggest is in part related to climatic variation. We estimate that input from phyllosphere N2 fixation over the study period contributes on average an additional 20% to the total daily N load per area within the seagrass meadow.
Similar content being viewed by others
Introduction
Eutrophication and ecosystem degradation as a result of nitrogen (N) pollution are among the greatest threats to the ecological integrity of coastal systems, and shallow lagoons with seagrass beds are particularly sensitive (Nixon et al. 2001; Howarth and Marino 2006; McGlathery et al. 2007). The trophic status, community structure, and ecological functioning of these ecosystems are heavily influenced by nutrient inputs and by climatic variables (McGlathery 2008). While many have studied the impact of changing external N inputs on shallow coastal ecosystems (for example, Valiela et al. 1992; Kennish et al. 2007; Wazniak et al. 2007), fewer have examined the importance of the potential N inputs from seagrass community N2 fixation to the ecosystem during the growing season relative to external N load, and how that might change over time as external nutrients loads increase or decrease (Cole and McGlathery 2012; Aoki and McGlathery 2019). Yet, biogeochemical processes that add or remove N within the seagrass bed are key factors in determining overall N availability and primary productivity.
Biological N2 fixation in seagrass-dominated ecosystems occurs both in sediments and on grass leaves colonized by diazotrophs, including cyanobacteria in epibiotic assemblages. The majority of studies in these systems have found that rates of heterotrophic fixation in the rhizosphere sediments are higher than fixation by epiphytes and that sediments are the dominant source of N2 fixation in seagrass beds (Welsh 2000 and references therein). However, some recent studies have found leaf-epiphyte N2 fixation rates on par with, or higher than, those for the sediment rhizosphere (see summary table in Carlson-Perret et al. 2018; Agawin et al. 2019; Carlson-Perret et al. 2019) and have concluded that phyllosphere N2 fixation can be a significant source of new N to support seagrass production, particularly when there are low external nutrient inputs (Capone et al. 1979; Cole and McGlathery 2012; Agawin et al. 2016). Additionally, the N input to seagrass communities from N2 fixation associated with leaf epiphytes can have dissimilar importance to the N demand for plant production and system metabolism (Capone et al. 1979).
As part of a long-term study on the effects of nitrogen on a seagrass-dominated coastal marine lagoon that has experienced a major increase in N load since the early 2000s, we have measured N2 fixation in most summers from 2005 to 2019. Nitrogen loading to the lagoon is primarily from groundwater inputs and creates a gradient of inorganic N concentrations between the inner and outer parts of the system. At the start of the study in 2005, we had hypothesized that rates of epiphytic N2 fixation would be relatively low and perhaps decrease over time due to the high nitrogen load, particularly in the areas closest to the high-N groundwater plume. Seagrass shoot-associated N2 fixation rates can vary with both leaf and bed age (Cole and McGlathery 2012; Agawin et al. 2016; Carlson-Perret et al. 2018), and there are almost no measurements reported in the literature within a particular system across multiple years. In this paper, we report data on N2 fixation associated with epiphytes on the seagrasses over more than a decade, the first such long term data we are aware of for a seagrass system and one of the very few studies on N2 fixation in the phyllosphere in a temperate system (see summary table in Carlson-Perret et al. 2018).
We used the acetylene reduction assay (ARA) to estimate nitrogen fixation, an indirect method widely used in many different ecosystems since being introduced in the late 1960’s (Stewart et al. 1967; Hardy et al. 1968). Despite the identified complications with ARA (Howarth et al. 1988a; Welsh 2000; Fulweiler et al. 2015; Aoki and McGlathery 2019), it is by far the most common method used in the literature to estimate N2 fixation associated with seagrasses (see summary tables in Welsh 2000, and Carlson-Perret et al. 2018; Agawin et al. 2019). Application of ARA data (acetylene reduced to ethylene) to extrapolate to N2 fixed requires the assumption of a conversion ratio, which varies in natural systems from the theoretical. To determine the conversion ratio, we calibrated our ARA measurements using two different methods. We ran a parallel assay using the 15N2-addition method to measure incorporation of gaseous N2 into organic N biomass (Montoya et al. 1996; Wilson et al. 2012), and we employed a novel application of the N2:Ar method (Kana et al. 1994) using membrane inlet mass spectrometry to measure removal of N2 from solution.
Our study system, West Falmouth Harbor on Cape Cod, MA (USA), has a large input of nitrate from a contaminated groundwater plume originating from a municipal wastewater treatment plant. The N input from this source was relatively steady over the 15-year period of this study, and there has been no concomitant increase in phosphorus, since phosphorus from the treatment plant effluents was trapped in the soil before reaching the harbor (Hayn et al. 2014, and manuscript in prep). We have observed marked changes in the seagrass habitat over the past decades, including the complete disappearance of the seagrass beds in the area where the plume enters the harbor. This unintentional ecosystem experiment provided an ideal opportunity to study the consequences of increased N loading along a spatial gradient on ecological function and biogeochemical cycling at the scale of an entire ecosystem for a coastal seagrass system. Here, we present data on how a moderate to high inorganic N load affects N2 fixation rates in the seagrass phyllosphere, how variable peak season rates are over a decadal time scale, and how significant the N input to the seagrass system from N2 fixation might be relative to the external N load.
Materials and methods
Study site
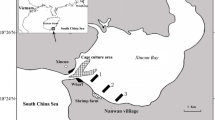
West Falmouth Harbor is a relatively shallow, tidally dominated lagoon with areas of dense beds of the temperate zone seagrass Zostera marina, also known as eelgrass (Fig. 1). Detailed site description data are provided in earlier publications (Berg et al. 2013; Hayn et al. 2014; Howarth et al. 2014). In this paper, we concentrate on two areas of the harbor, one site located in the inner, northeast portion of the harbor called Snug Harbor (SH) and the other site (outer harbor, OH) located further west, just inside the inlet that exchanges coastal ocean water from Buzzards Bay. Snug Harbor is more nitrogen-enriched than the OH both because the high N plume enters the harbor through the SH shoreline and because OH has a shorter residence time due to its proximity to the outlet with Buzzards Bay. The average N load to all of West Falmouth Harbor is 4.2 mmol m−2 d−1, while SH receives an estimated 17 mmol m−2 d−1 (Howarth et al. 2014). The whole-harbor N load is near the average for small estuaries in southern New England, most of which are nutrient-enriched to some extent, while the load to the SH sub-basin is at the very high end observed in this region (Latimer and Charpentier 2010). Seagrasses have been almost entirely absent in SH following a near-complete die-off event during late summer 2010; sparse shoots were seen in spring 2011, but the bed has been absent since (Fig. 1).
Location of our sampling sites in West Falmouth Harbor, and location of the harbor adjacent to Buzzards Bay on Cape Cod (MA) in northeastern US. Routine annual sampling sites are shown by red stars in Outer Harbor and Snug Harbor. Other sampling sites shown by black dots. Extent of seagrasses in 2018 shown in dark green. Seagrasses occurred in Snug Harbor until 2011, indicated by green stripes, but have been absent since
As part of a larger study of coupled biogeochemical cycles of carbon, nitrogen, and phosphorus in this system (Ganju et al. 2012; Hayn et al. 2014; Howarth et al. 2014), we measured water column temperature and salinity using YSI data sondes deployed continuously during the summer months in each basin (Howarth et al. 2014) from 2005 to 2019. Temperature and salinity during the study period varied with the tide; temperatures were not significantly different at the two sites, and salinities were slightly lower in SH (Table 1). We also measured concentrations of water column dissolved inorganic nutrients [nitrate + nitrite, ammonium, soluble reactive phosphorus (SRP)] one or more times each year during July and early August from 2006 to 2016 hourly over a 24-h period, capturing two full tidal cycles. Details of sampling and analyses are as given in Hayn et al. (2014). All inorganic nutrient concentrations are low during the summer months (Table 1). Concentrations of inorganic N are higher in SH (NH4+: p < 0.001, NO2− + NO3−: p < 0.001), reflecting its proximity to the groundwater N load. Concentrations of SRP are higher in the OH (p < 0.001), reflecting an ocean source that enters the harbor from Buzzards Bay (Hayn et al. 2014; Howarth et al. 2021). The ratio of DIN:SRP over the years sampled are consistently very low in both OH and SH, with medians of 0.6 (OH) and 1.5 (SH).
Sampling protocol
We characterized N2 fixation by epiphytes growing on Zostera blades every summer during the peak growing season from 2005 to 2010 in both SH and OH and from 2011 to 2019 in OH. Measurements were made at the same sites between July 20 and 30 in every year except 2006, when we sampled on July 12. We have found from more detailed temporal measurements made in 2007, 2008, and 2010 (data not shown) that epiphytic N2 fixation rates are low until late June into early July, and peak in mid-July through mid-August, so we sampled annually within the latter period to capture peak rates of N2 fixation. Additionally, we simultaneously sampled at 18 sites across the OH in 2019 (Fig. 1) to ascertain the extent of spatial variation in rates of N2 fixation. For each sampling, we collected 20 to 30 randomly selected shoots at each site by SCUBA diving, excluding the smallest shoots with no visible epiphyte cover. Plants were placed in zip-top bags taking care to minimize the disturbance to epiphytes, transported in a cooler back to the laboratory at ambient temperature, and assayed for N2 fixation the same day.
Shoot density in both sampling areas was assessed during most years of the study; measurements were made in July using a 0.25 m2 quadrat (n = 3) at 15 sites distributed throughout the OH seagrass bed and 10 sites in the SH bed to allow scaling of the phyllosphere N2 fixation rates to an areal estimate. Shoot density showed very little inter-annual variation (R2 = 0.009, P = 0.48); for our calculations we use the mean values across all survey stations at each site and all years of survey data (Table 1). From two years of detailed size class measurements (Online Resource 1, Text S1), we saw on average across the bed that 43% of shoots were of the smallest size with few visible epiphytes. To provide a conservative estimate of epiphytic N2 fixation rate per m2 of seagrass bed, we assume that these plants fix minimal N and modify our total shoot density to only represent larger shoots similar to those we used in our assay.
Acetylene reduction assay
We used the acetylene reduction assay (ARA) to routinely estimate rates of N2 fixation in the phyllosphere of Zostera marina and the water column, following the general approach described in Howarth et al. (1993) and Marino (2001) for planktonic N2 fixation, and Reynolds et al. (2015) for N2 fixation and biomass associated with seagrass blade epiphytes. For our standard runs at each site, we randomly selected intact shoots and cut the top 15 cm of the second and fourth blades (by age, from youngest) and placed these in individual 120-ml serum bottles containing 90 ml of 0.2 µm-filtered seawater collected at the sampling sites, being careful not to disturb the attached epiphytes. Blades 2 and 4 were chosen as representative of the whole shoots, which at our sites typically had 4–5 intact blades (Table 1); the top 15 cm (i.e. oldest portion) of a blade was the area most colonized by epiphytes. We measured the length and width of each blade on each shoot (Table 1) and calculated single-sided area for the incubated portions. Four replicates of each blade and treatment (light, dark) were generally set up for each sampling site. Acetylene sufficient to saturate the nitrogenase enzyme (10% vol/vol final; Flett et al. 1976) was added to each incubation bottle as 10 ml of an acetylene-saturated seawater or artificial seawater solution (salinity-matched to the sites). Immediately after the acetylene addition, the sample bottles were sealed with crimped septa, gently mixed, and then incubated for 4 h in a water bath held at ambient field temperature in a Conviron PGR15 growth chamber, equipped with metal halide and sodium lamps to give a full PAR spectrum.
In 2016, we evaluated the sensitivity of the rate of N2 fixation (as ethylene production) to different levels of light in the incubation. Layers of screen were wrapped around the incubation bottles and light inside was measured with Onset Hobo Pendant loggers UA-002-64 to determine shading levels. Nitrogen fixation on blade four of seagrass shoots from the OH site was assayed using our standard ARA methodology as described above at shading levels that allowed through 10%, 20%, 55% or 100% of ambient light in the growth chamber at a PAR of approximately 1000 µE m−2 s−1. For all of the routine ARA measurements, the samples were incubated in the dark and or in the light at 550 to 750 µmol m−2 s−1, measured with a LiCor LI-1000 and LI-192 PAR sensor.
Incubations were stopped by placing the bottles in coolers filled with an ice-water bath, as in previous studies (Howarth et al. 1993; Reynolds et al. 2015). We verified for these assays that placing the bottles in ice-water was as effective for quickly stopping nitrogenase enzyme activity as poisoning with ZnCl2 (data not shown). Bottles were kept at 0 ℃ until assayed for ethylene (within 48 h) using a Shimadzu GC-14a gas chromatograph with a flame ionization detector and an 80/100-mesh Porapak N column; see Online Resource 1 (Text S2) for further detail on the GC analysis. Time-zero ethylene blanks prepared over the course of the assay in the same way as the samples were subtracted from the ethylene production at the end of the incubation; blanks were always below 0.6 nmol ethylene per bottle. Over the multiple years of data, we estimate the limit of detection for this ARA assay as between 2 and 10 nmol of ethylene per liter, corresponding to less than 0.035 nmol ethylene cm−2 h−1 when expressed per surface area of blade incubated, for a blade of average width. We also ran controls to assess ethylene production by blades with epiphytes with no added acetylene, and for abiotic ethylene production (Wilson et al. 2012) in incubations with filtered site water and no plants. No significant ethylene production was observed in any of these incubations (data not shown). Further, we added ethylene at a concentration typically produced over our ARA incubations to bottles with blades but without acetylene to determine if consumption of ethylene by hydrocarbon-oxidizing bacteria might be occurring, which could result in an underestimation of acetylene reduction; no significant reduction in the time zero ethylene spike was detected (data not shown).
Calibration of ARA
We empirically determined the conversion ratio for rates of acetylene reduction to moles of N fixed during our routine site measurements in July of 2018 by comparing ethylene production with N2 fixation determined two other ways: (1) from a decrease in dissolved N2 during an incubation as measured by the ratio of dissolved N2 to Ar (mass 28 to mass 40) on a membrane inlet mass spectrometer (MIMS, Kana et al. 1994), and (2) from uptake of 15N-labelled N2 into biomass (Montoya et al. 1996). For each of the three assays, we used 10 replicates of a homogenized slurry of epiphytes rather than intact blades with epiphytes as used for our routine ARA. This was necessary because we found that the diffusion of gases from lacunae in the seagrass blades gives erroneous results for the change in N2:Ar. Additionally, to test for effects on N2 fixation rate due to the disruption of the epiphyte-plant interface, such as loss of anoxic microzones or other over-exposure to oxygen that can poison the nitrogenase enzyme in diazotrophs that do not have heterocysts (Bothe 1982), we ran an ARA on the top 15 cm of grass blades as described for our routine assay at the same time as aliquots of the slurry containing a similar epiphyte biomass. All samples from each method used were incubated under the same conditions as described above for the routine ARA runs.
The MIMS N2:Ar method measures the net change in dissolved N2 gas in solution over the incubation period. Sample conditions were highly unfavorable to denitrification (very low DIN, oxygenated water) so we can assume that the changes in N2 over the incubation were almost entirely driven by N2 fixation. Replicate samples were prepared by slowly adding 5 ml of the epiphyte slurry to 6.5 ml of atmosphere-equilibrated, filtered site water in 12 ml exetainers and carefully capping the tubes to ensure no headspace or bubble entrainment. Incubations were ended by injecting ZnCl2 solution and samples were stored underwater until analysis on a Bay Instruments MIMS. Additional replicates for time-zero N2:Ar were prepared as above, with immediate addition of ZnCl2. For the 15N-labelled N2 uptake into biomass assay, replicate samples were set up in serum bottles containing 5 ml of the epiphyte slurry and filtered seawater such that they were filled and capped with no headspace. Ten ml of 15N2-enriched water solution prepared by equilibrating 15N2 gas (98+ %, Cambridge Isotope Labs) in filtered seawater for 24 h was injected into each bottle using a long syringe needle and a shorter vent needle to release pressure. Incubations were ended by filtration onto pre-ashed GF/F filters, dried, and analyzed at the Marine Biological Laboratory Stable Isotope Facility for δ 15N in the biomass. We used a bootstrap analysis (Efron and Tibshirani 1993) to calculate the ratio of ethylene produced (ARA) to N2 fixed for each of the intercalibration assays. Full details of the intercalibration experiments and methodologies used are given in Online Resource 1 (Text S3).
Results and discussion
Determination of conversion ratio for ARA to N 2 fixed
The acetylene reduction assay compared very well with both the 15N incubation and the MIMS analysis that directly measured the decrease in the N2:Ar ratio, when analyzed relative to the theoretical ratio between acetylene reduced to ethylene and N2 fixed (Fig. 2). We consider the N2:Ar method result to be the most robust of the three measurements as it directly measures the decrease in dissolved N2 gas due to N2 fixation. Acetylene reduction is an indirect assay, and the 15N approach could underestimate N2 fixation, to the extent that dissolved organic 15N-labelled products were not measured on our filtered samples. Nonetheless, the difference between the MIMS and 15N results were small, as discussed in more detail below. It is important to note that acetylene reduction measured on the epiphyte slurry we used with this test was quite comparable to that measured on intact plant-epiphyte assemblages when normalized to epiphyte biomass (Online Resource 1, Figure S1), indicating that N2 fixation rates weren’t significantly affected by the removal of the epiphyte assemblage from the seagrass blade surface.
Calibration results showing rates per epiphyte biomass, as measured by.15N2 addition, by decrease in N2 as measured by N2:Ar (MIMS), and by acetylene reduction (ARA). Horizontal lines represent median values, boxes show the middle two quartiles, and black dots show individual measurements. Y axes are scaled to a 4.5:1 ratio for ethylene to N2
A simple comparison of the mean results of the acetylene reduction assay with the N2:Ar method suggests a ratio of 4.1:1 ethylene to N2. However, the comparison of the means for our results may not be appropriate since the MIMS data are not normally distributed, as seen by the range of replicate values shown in Fig. 2. We have no reason to discount any of the data. Therefore, a bootstrap analysis approach was used to calculate the ratio of ethylene produced (ARA) to N2 fixed for each of the other concurrent assays (details in Online Resource 1, Text S3). We find the best estimate for the ethylene to N2 conversion ratio in our system, taking into account the variation in both assays, is 4.5:1, (95% confidence limit of 3.7:1 to 5.1:1, Online Resource 1, Figure S2), from the comparison with the N2:Ar method (MIMS) data as discussed above. This empirically determined relationship is very close to the theoretical ratio of 3:1 to 4:1 for the stoichiometric relationship between acetylene reduction to ethylene and N2 reduction to ammonium often used in the literature to convert ARA data. These conversion ratios are based on the relative electron transfers for the two reactions (3:1) and with additional consideration of proton reduction to H2 that would not occur with acetylene reduction (4:1) (Capone 1988; Welsh 2000). The ratio for acetylene reduced to N2 fixed that we calculate using the 15N incubations was slightly higher, at 5.3:1, with a 95% confidence limit of from 4.9:1 to 5.8:1 (Online Resource 1, Figure S3).
There are few other intercalibrations of ARA for seagrass ecosystems; all use 15N2 incorporation into biomass, and none include the blade epiphyte component. Mean acetylene reduction to 15N2 fixation ratios reported in the seagrass literature are 2.6 to 4.6, with a range of 0.8 to 15.4 (Patriquin and Knowles 1972; O’Donohue et al. 1991; Welsh 2000). In this paper, we convert the acetylene reduction measurements to rates of N2 fixation using the ratio of 4.5:1 ethylene:N2 (2.25 mol ethylene per mole N), based on these direct empirical results from our system. We note that this ratio is slightly higher than the theoretical ratios used in nearly all seagrass N2 fixation studies using acetylene reduction in the literature (summary tables in Welsh 2000 and Carlson-Perret et al. 2018), which will result in our N2 fixation estimates being 12% to 33% lower than studies using the theoretical ratios discussed above.
Rates of N2 fixation
The light gradient experiment demonstrated that the rate of acetylene reduction associated with the seagrass blade epibiont assemblage in our system is quite unresponsive to light intensities in the range between 100 and 1000 µmol m−2 s−1 (Fig. 3), indicating that the light levels for the incubations were sufficient to saturate the light requirement of the nitrogen-fixing organisms. Field light levels in OH at 0.8 m above the sediment surface (the approximate top of the seagrass canopy across the tidal range) averaged 860 µE m−2 s−1 during mid-day according to a field survey in 2012 (Dickhudt et al. 2012; del Barrio et al. 2014). From those data, we estimate that in July, light levels are above 100 µE m−2 s−1 for between 10.2 and 13.0 h per day, with a mean of 12.0 h per day. We use this value to scale our hourly rates to daily estimates and to make comparisons with other published values. Ambient water from both sites incubated under both light and dark conditions in parallel with the seagrass blade measurements consistently showed very low water column N2 fixation, at or below the detection limit of our assay.
In Fig. 4 we show rates of ethylene production per hour per area of seagrass blade in the dark and in the light for both SH and OH through 2010, the last year we were able to measure epiphytic N2 fixation in the former before the loss of seagrasses there. One striking aspect is that rates are much higher in the light. Integrating the light and dark measurements over a 24-h period as described above, N2 fixation in the light accounts for a mean of 95% and a median of 96% of the total daily rate (Online Resource 1, Figure S4). This stands in contrast with some previous studies on N2 fixation in the seagrass phyllosphere, and particularly for a temperate system, where the only other studies with Zostera marina that separated light and dark incubations (both using ARA) found either no difference in activity in the light or the dark (McRoy et al. 1973, North Carolina and Alaska) or 99% of the total activity in the dark and attributed to heterotrophic bacteria (Cole and McGlathery 2012, Virginia). In sub-tropical and tropical systems, where most of the published studies of N2 fixation associated with seagrass epibionts have been done, a range in the relative importance of light and dark rates have been found. Some studies show a strong predominance of phyllosphere N2 fixation estimated in the light (Capone et al. 1979; Carlson-Perret et al. 2019) while others found similar rates in the light and dark, suggesting that the community of diazotrophs can be complex and diverse and not dominated by a single group (Agawin et al. 2016).
Our much higher rates in the light were presumably due to cyanobacteria that more actively fix in the light. While we did not systematically characterize the epiphytic community in West Falmouth Harbor, a qualitative survey found cyanobacteria capable of fixing N were common, including both non-heterocystous (Lyngbya spp.) and heterocystous (Anabaena spp.) species (J. O’Neill, personal communication). While some non-heterocystous, filamentous marine cyanobacteria typically fix N in the dark to avoid O2 inactivation of nitrogenase (Griffiths et al. 1987), some Lyngbya sp. have been found to fix at substantial rates predominantly in the light (Jones 1990). The time scale of influence of diel light–dark cycling and other environmental conditions on N2 fixation rates in situ is likely somewhat dependent on the energetics and adaptations of the specific diazotrophs present (Griffiths et al. 1987). Such physiological parameters can vary on the scale of minutes to hours, the same time frame as rate measurements such as those reported here are typically made (Marcarelli et al. 2022). As such, in the absence of very detailed temporal sampling and spatial sampling, assumptions such as those we use above are required in order to make estimates of daily N2 fixation rates and their significance to ecosystem N inputs.
Nitrogen fixation (as ethylene production) rates are significantly higher in SH than in OH in several years but not all, for both younger and older blades (Fig. 4). Variability across replicate samples was generally highest in the years when the N2 fixation rates are high (2007, 2008, 2010). For the older, more heavily colonized blade 4, the difference between SH and OH rates was highly significant (t-test, p = 0.02) in 2008 and 2010 and significant in 2007 (p = 0.11), but p values were > 0.5 in the years where N2 fixation rates were low in SH (2005, 2006, 2009). The younger blade 2 had significantly greater N2 fixation rates in SH compared to the OH in every year except 2005 (p = 0.02). An obvious question is whether or not our regular sampling sites in SH and OH were more broadly representative of the plant-associated N2 fixation activity over the whole bed area. We cannot answer this with certainly for the more eutrophic SH site, where seagrasses have been absent since 2011. For OH, the spatial survey of N2 fixation done concurrently with the routine measurement at the regular OH site in 2019 gives us confidence that our site selection is representative. As shown in Online Resource 1 (Figure S5), the median rate of ethylene production and the variability at our long-term OH sampling site is well within the variation and middle 50% of measurements across individual blades sampled at 18 other sites in the Outer Harbor seagrass bed (Fig. 1).
Daily rates of N2 fixation per area of blade measured on blades 2 plus 4 across all 14 years that we have data are shown in Fig. 5. The light and dark ARA hourly data were combined using a 12-h light–dark cycle as described above, and converted from ethylene produced to N fixed using the ARA calibration 2.25:1 (ethylene:N) molar ratio. After 2011, there were virtually no seagrasses in SH and therefore no further estimates of N2 fixation in the phyllosphere at that site. There appears to be a jump up in the OH rates after the loss of the grass beds in SH, and then a slight trend of decreasing rates of N2 fixation in OH after 2013/14, which we explore in more detail in the discussion below (see Biogeochemical and Ecological Influences).
Rates of nitrogen fixation expressed per area of leaf per day (for the top 15cm of leaf length), for younger blades (blade 2) and older blades (blade 4) in Outer Harbor (a) and Snug Harbor (b) from 2005–2019. Error bars represent the standard error of replicates each year. Note that the y axis scale in (b) is 2 × that in (a)
Comparison of rates to other studies
To compare our data with other published rates of epiphytic N2 fixation and with the N load to the harbor, we scale the daily rates we measured per area of seagrass blade over the top 15 cm of blades 2 and 4 (Fig. 5) to rates per shoot and to areal rates expressed per square meter of seagrass bed (Fig. 6). We conducted several detailed experiments to determine scaling factors, and used these to calculate rates in mmol N m−2 day−1 (Online Resource 1, Text S1 and equations S1 and S2). Applying these scaling calculations to our measurements, we estimate that the average rate of N2 fixation by epiphytes on plants in OH across the multiple years of our study is 3.6 µmol N d−1 per shoot, and 5.5 µmol N d−1 per shoot in SH. Applying the annual shoot densities measured as described above, the mean N2 fixation by epiphytes in OH over the entire study is 0.94 mmol N m−2 d−1, expressed per area of seafloor within the seagrass bed (Fig. 6); annual estimates range from 0.1 mmol N m−2 d−1 (2006) to 1.9 mmol N m−2 d−1 (2014). Values in SH are somewhat higher, up to 2.6 mmol N m−2 d−1 (2008), although we have fewer years of field data in SH with which to scale the N2 fixation rate at that site. See Online Resource 1 (Text S1) for full calculation details.
Epiphyte-associated N fixation rate in the OH (gray) and SH (black), expressed per area of seagrass bed. Error bars represent the standard error, propagated from the SE of the input data (Nichols 2017)
The N2 fixation rates measured in this study range from moderately to very high as compared to most other published studies of N2 fixation in the phyllosphere of seagrasses, and particularly so for studies in temperate estuarine systems that typically receive relatively high N inputs, where reported rates range from 0 to 0.19 mmol N m−2 d−1 (McRoy et al. 1973; Lipschultz et al. 1979; Welsh 2000). The highest mean rate of epiphytic N2 fixation previously reported in a temperate system is 0.36 mmol N m−2 d−1, in a shallow coastal bay system with extremely low external N inputs (Cole and McGlathery 2012). In sub-tropical and tropical seagrass ecosystems, which are often thought to have higher rates of N2 fixation, published rates for the seagrass phyllosphere vary from 0.016 to 1.1 mmol N m−2 d−1 (Capone and Taylor 1977; Capone et al. 1979; Iizumi 1992; Moriaty and O’Donahue 1993; Hamisi et al. 2009; Agawin et al. 2016; Carlson-Perret et al. 2018; Agawin et al. 2019; Carlson-Perret et al. 2019), with the exception of one exceptionally high measurement in a very early study using ARA (Goering and Parker 1972). The mean rate of epiphyte-associated N2 fixation at our OH site over the 14 years of measurement (0.94 mmol m−2 d−1) is comparable to the high end of the range reported in the literature.
Biogeochemical and ecological influences
Our original hypothesis when we started this long-term study was that the high inorganic N load would result in low rates of epiphytic N2 fixation across West Falmouth Harbor, but particularly in SH where most of the N load enters. Nitrogen fixation by cyanobacteria is an energetically expensive process (Bothe 1982), and so we expected that the high N load would diminish the need for nitrogenase synthesis and N2 fixation to fuel primary production. Surprisingly, we found that N2 fixation rates per shoot in SH were moderate to quite high during some years and remained so despite the large external N input. It is important to note that although the N load to SH is high, the water column DIN concentration is relatively low (Table 1), and as such is unlikely to significantly inhibit nitrogenase synthesis and activity (Knapp 2012; Howarth et al. 1988b; Vitousek et al. 2002). Presumably DIN remains low due to rapid uptake by primary producers and net loss from the sediments through denitrification (Aoki et al. 2020). The DIN:SRP molar ratios in both SH and OH are well below the 16:1 threshold for N limitation as generally established by the Redfield ratio, and the DIN:TP molar ratios are below the 2:1 to 8:1 threshold found in the coastal Baltic Sea (Ptacnik et al. 2010). Thus, conditions overall in both OH and SH are favorable for N fixation, and perhaps more strongly so in the Outer Harbor. Yet the highest rates of epiphytic N2 fixation per blade area were observed in SH during several years prior to the seagrass die-off there (Figs. 4 and 5).
This SH–OH difference cannot be explained on the basis of inorganic nutrient concentrations, since DIN concentrations and the DIN:SRP ratio are higher in SH than OH, and the SRP concentrations are lower (Table 1). One would expect these nutrient factors to lead to relatively lower N2 fixation in SH (Howarth et al. 1988a, b; Vitousek et al. 2002). So then, what might explain the observed higher N2 fixation at the Snug Harbor site? In addition to relative and absolute nutrient availability, controls on colonization and the biomass of N2-fixing organisms, such as grazing pressure, can have strong influences on ecosystem N2 fixation (Vitousek et al. 2002). Using a reciprocal transplant experiment, we demonstrated a strong effect of grazing on epiphytic N2 fixation rates in this system (Reynolds et al. 2015). When shoots were transplanted from SH to OH, epiphyte biomass and rates of N2 fixation rapidly declined over 6–12 days, while transplanting shoots from OH to SH resulted in a rapid fivefold increase in rates of epiphytic N2 fixation. The density of invertebrate animals that graze on epiphytes was 4 times higher in OH than in SH, and epiphyte biomass at the SH site was on average 3 times higher than in the OH (Reynolds et al. 2015). In that study, the authors postulated that poorer quality environmental conditions in the bottom water, such as lower median oxygen concentrations, and/or in the sediment in SH as compared to OH strongly influenced the lower grazer abundance and indirectly resulted in the higher rates of N2 fixation observed in SH. Regarding sediment quality changes relevant to this study, before the die-off of Zostera in SH, dissolved sulfide concentrations in the top 5 cm of sediments averaged 1.6 mM, a level 5 to 20 times greater than that observed in OH and likely to be toxic (Haviland et al. 2022).
The abrupt loss of the seagrasses in SH created a sort of natural experiment that allowed us to look at long-term changes to the down-gradient site associated with the loss of significant primary producers in SH. After the seagrass die-off in SH in 2011, rates of epiphytic N2 fixation appear to have risen distinctly in OH (Fig. 5). We suspect that the loss of seagrasses in SH may have decreased the retention of the large quantity of DIN entering the harbor there, perhaps leading to increased eutrophication in OH. In a parallel study, Haviland et al. (manuscript in prep.) have found that sediment organic matter has increased more than threefold and dissolved sulfide concentrations more than fivefold in OH by 2022 compared to the years before seagrass die-off in SH. These changes could have adversely affected the abundance of animals that graze on epiphytes. Although we have no direct data on changes in macroinvertebrate grazers over the duration of our study, in 2008–2009 the OH had an average of 0.5 mg cm−2 total epiphyte biomass on the surface of seagrass blades measured on whole plants (Reynolds et al. 2015), while Haviland et al. (2022) found in 2018–2019 that total epiphyte biomass had increased sixfold to an average of 3 mg cm−2 in the same area of the harbor. In both studies, between one third and one half of the total epiphyte biomass was organic (as ash-free dry weight). The increase in epiphyte biomass in the OH supports the idea that there has been a reduction in grazing pressure and an increase in epiphyte-associated N2 fixation consistent with the response to degraded benthic habitat conditions as observed in the Reynolds et al. (2015) study. Note that rates of epiphytic N2 fixation at the OH site are correlated with epiphytic biomass in years where both were quantified (2016 through 2019; Online Resource 1, Figure S7, R2 = 0.34, P < 0.001). We tentatively conclude that the interaction of bottom up (higher N reaching the OH after the seagrass die-off) and top-down (decreased grazing pressure) factors are likely linked with the observed increase in epiphytic N2 fixation in OH by 2013.
Rates of N2 fixation in both SH and OH show a large inter-annual variation (Fig. 6), suggesting a key role for environmental drivers such as climatic variation in addition to more slowly changing bottom up or top-down controls. Rates are correlated with the average daily photosynthetically active radiation (PAR) for the time period from May 1 to the sampling date later in the summer each year in both OH (Fig. 7; R2 = 0.43, P = 0.007) and SH (Online Resource 1, Figure S8; R2 = 0.47, P = 0.08); see Online Resource 1 (Text S4) for PAR measurement details. Further, our N2 fixation rates are directly correlated with epiphyte biomass at the time of our measurements (Online Resource 1, Figure S7), suggesting that factors influencing growth in the spring and early summer are important controls on peak-season fixation rates.
Higher light availability during the spring and early summer period can have impacts on both seagrass morphometry and epiphyte colonization rates that are likely to influence total epiphyte biomass and perhaps community composition during the peak summer period. Similarly, higher solar radiation early in the growing season may lead to warmer water temperatures that in turn favor more rapid growth of algae and inclusion of cyanobacteria in the epiphyte community (Marcarelli and Wurtsbaugh 2006). The water residence time in West Falmouth Harbor is short (1 to 2 days, Ganju et al. 2012; Howarth et al. 2014), so solar heating would need to occur at the regional scale in order to influence the temperature in the harbor. Indeed, continuous temperature data taken nearby in Woods Hole (which reflect the broader regional temperature of Buzzards Bay) show a correlation with PAR over the time period each year from May 1 to our July sampling (Online Resource 1, Figure S10; R2 = 0.52, P = 0.003). Over this same time period, rates of N2 fixation in the OH for the 14 years of measurements are also correlated with the regional water temperature (Online Resource 1, Figure S11; R2 = 0.21, P = 0.06). Since PAR and water temperatures are correlated, we cannot distinguish between these two factors. It is possible that either one or both could be contributing to the higher epiphytic N2 fixation rates observed at both sampling sites in years with higher seasonal PAR, interacting with the differences in bottom up and top-down controls discussed above. This apparent climate response suggests the value of further mechanistic investigation.
Importance of phyllosphere N2 fixation to the N economy of West Falmouth Harbor
The daily mean N inputs to the seagrass meadows during the summer season from epiphytic N2 fixation are significant relative to the external N inputs to West Falmouth Harbor, estimated as 4.2 mmol N m−2 d−1 (Hayn et al. 2014; Howarth et al. 2014). Our mean annual N2 fixation rate estimated in the OH of 0.94 mmol N m−2 d−1 is a 21% increase above this already high external load, per area within the seagrass beds. The highest rates we observed during this study, measured in SH in 3 of the 4 years just prior the loss of the beds there, added an estimated average 1.9 mmol N m−2 d−1, or 45% to the total N load to the seagrass bed. Of course, the external N load continues year-round, while significant epiphytic N2 fixation occurs only for a limited time period each year in the harbor. Still, the N input from N2 fixation likely considerably increases the N available to the primary producers during the peak-growth summer period. The scope of the study does not allow us to evaluate the fate of this fixed N and so to elucidate the complete role of N2 fixation in the N cycle in West Falmouth Harbor ecosystem. Nonetheless, the additional N inputs to the seagrass meadows during the active growing season from N2 fixation are significant, and represent an unexpected increased feedback with nutrient enrichment that could contribute to exacerbating eutrophication.
Concluding thoughts
The moderate to high N2 fixation rates in the phyllosphere in WFH despite a high inorganic N load are contrary to current conceptual models based on nutrient stoichiometric considerations, particularly since we observed the highest rates at the more directly N-impacted site. The high variability in phyllosphere N2 fixation rates both within and across the two long-term study sites is likely a result of a complex interaction of bottom up (nutrient), top down (grazer), and climatic influences. Our results illustrate the value of an ecologically-based consideration of controls and constraints on N2 fixation to provide a more complete understanding of the rates and dynamics of biological N2 fixation in shallow seagrass ecosystems, as in terrestrial and aquatic systems generally (Vitousek et al. 2002; Marcarelli et al. 2022). Further, this study demonstrates the potential importance of the role biological N2 fixation plays in the overall N economy and health of seagrass ecosystems, and underscores the value of long-term observations toward understanding the complicated feedbacks present in shallow lagoonal systems such as West Falmouth Harbor that are undergoing dramatic shifts as external drivers such as nutrient loading and climatic conditions change.
Data availability
The supporting datasets generated during and /or analyzed during the current study will be available after publication in the EDI data repository and accessible via https://www.research.howarthlab.org/WFH.php.
References
Agawin NSR, Ferriol P, Cryer C, Alcon E, Busquets A, Sintes E, Vidal C, Moya G (2016) Significant nitrogen fixation activity associated with the phyllosphere of Mediterranean seagrass Posidonia oceanica: first report. Mar Ecol Prog Ser 551:53–62. https://doi.org/10.3354/meps11755
Agawin NSR, Ferriol P, Sintes E (2019) Simultaneous measurements of nitrogen fixation in different plant tissues of the seagrass Posidonia oceanica. Mar Ecol Prog Ser 611:111–127. https://doi.org/10.3354/meps12854
Aoki L, McGlathery KJ (2019) High rates of N fixation in seagrass sediments measured via direct 30N2 push-pull method. Mar Ecol Prog Ser 616:1–11. https://doi.org/10.3354/meps12961
Aoki L, McGlathery KJ, Oreska MPJ (2020) Seagrass restoration reestablishes the coastal nitrogen filter. Limnol Oceanogr 65(1):1–12. https://doi.org/10.1002/lno.11241
Berg P, Long MH, Huettel M, Rheuban JE, McGlathery KJ et al (2013) Eddy correlation measurements of oxygen fluxes in permeable sediments exposed to varying current flow and light. Limnol Oceanogr 58(4):1329–1343. https://doi.org/10.4319/lo.2013.58.4.1329
Bothe H (1982) Nitrogen fixation. In: Carr NG, Whitton BA (eds) The Biology of Cyanobacteria. University of California Press, Berkeley, pp 87–104
Capone DG, Taylor BF (1977) Nitrogen fixation (acetylene reduction) in the phyllosphere of Thalassia testudium. Mar Biol 40:19–28
Capone DG, Penhale PA, Oremland RS, Taylor BR (1979) Relationship between productivity and N2 (C2H2) fixation in a Thalassia testudinum community. Limnol Oceanogr 24(1):117–125
Carlson-Perret NL, Erler DV, Eyre BD (2018) Dinitrogen (N2) fixation rates in a subtropical seagrass meadow measured with a direct 15N–N2 tracer method. Mar Ecol Prog Ser 605:87–101. https://doi.org/10.3354/meps12755
Carlson-Perret NL, Erler DV, Eyre BD (2019) Comparison of dinitrogen fixation rates in two subtropical seagrass communities. Mar Chem 209:62–69. https://doi.org/10.1016/j.marchem.2019.01.002
Cole LW, McGlathery KJ (2012) Nitrogen fixation in restored eelgrass meadows. Mar Ecol Prog Ser 448:235–246. https://doi.org/10.3354/meps09512
del Barrio P, Ganju NK, Aretxabaleta AL, Hayn M, García A, Howarth RW (2014) Modeling future scenarios of light attenuation and potential seagrass success in a eutrophic estuary. Estuar Coast Shelf Sci 149:13–23
Dickhudt PK, Ganju NK, Montgomery ET, Martini MA (2012) Oceanographic and water-quality measurements-- West Falmouth Harbor, Massachusetts, 2009, 2010, 2012: U.S. Geological Survey data release. https://stellwagen.er.usgs.gov/wfal.html.
Efron B, Tibshirani R (1993) An Introduction to the Bootstrap. Chapman & Hall/CRC, Boca Raton
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2004) Reply to comment on our paper “Comparison of isotope pairing and N2: Ar methods for measuring sediment denitrification.” Estuaries 27:177–178
Flett RJ, Hamilton RD, Campbell NER (1976) Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol 22:43–51
Fulweiler RW, Heiss EM, Rogener MK, Newell SE, LeCleir GR, Kortebein SM, Wilhelm SW (2015) Examining the impact of acetylene on N-fixation and the active sediment microbial community. Front Microbiol 6:1–9
Ganju NK, Hayn M, Chen S, Howarth RW, Dickhudt PJ, Aretxabaleta AL, Marino R (2012) Tidal and groundwater fluxes to a shallow microtidal estuary: constraining inputs through field observations and hydrodynamic modeling. Estuaries Coasts 35:1285–1298. https://doi.org/10.1007/s12237-012-9515-x
Goering JJ, Parker PL (1972) Nitrogen fixation by epiphytes on sea grasses. Limnol Oceanogr 17(2):320–323
Griffiths MJ, Gallon JR, Chaplin AE (1987) The diurnal pattern of dinitrogen fixation by cyanobacteria in situ. New Phytol 107:649–657. https://doi.org/10.1111/j.1469-8137.1987.tb00903.x
Hamisi MI, Lyimo TJ, Muruke MHS, Bergman B (2009) Nitrogen fixation by epiphytic and epibenthic diazotrophs associated with seagrass meadows along the Tanzanian coast, Western Indian Ocean. Aquat Microb Ecol 57:33–42. https://doi.org/10.3354/ame01323
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185
Haviland KA, Howarth RW, Marino R, Hayn M (2022) Variation in sediment and seagrass characteristics reflect multiple stressors along a nitrogen-enrichment gradient in a New England lagoon. Limnol Oceanogr 67(3):660–672. https://doi.org/10.1002/lno.12025
Hayn M, Howarth R, Marino R, Ganju N, Berg P, Foreman KH, Giblin AE, McGlathery K (2014) Exchange of nitrogen and phosphorus between a shallow lagoon and coastal waters. Estuaries Coasts 37(Suppl 1):S63–S73. https://doi.org/10.1007/s12237-013-9699-8
Howarth RW, Marino R (2006) Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: evolving views over three decades. Limnol Oceanogr 51:364–376
Howarth RW, Marino R, Lane JM, Cole JJ (1988a) Nitrogen fixation in freshwater, estuarine, and marine ecosystems. l Rates and Importance. Limnol Oceanogr 33:669–687
Howarth RW, Marino R, Cole JJ (1988b) Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 2. Biogeochemical controls. Limnol Oceanogr 33:688–701
Howarth RW, Marino R, Butler T, Lunde K, Swaney D, Chu CR (1993) Turbulence and planktonic nitrogen fixation: a mesocosm experiment. Limnol Oceanogr 38:1696–1711
Howarth RW, Hayn M, Marino RM, Ganju N, Foreman K, McGlathery KJ, Giblin AE, Berg P, Walker JD (2014) Metabolism of a nitrogen-enriched coastal marine lagoon during the summertime. Biogeochemistry 118:1–20. https://doi.org/10.1007/s10533-013-9901-x
Howarth RW, Chan F, Swaney DP, Marino RM, Hayn M (2021) Role of external inputs of nutrients to aquatic ecosystems in determining prevalence of nitrogen vs. phosphorus limitation of net primary productivity. Biogeochemistry 154:293–306. https://doi.org/10.1007/s10533-021-00765-z
Iizumi H (1992) Acetylene reducing activity at a tropical seagrass bed in Papua New Guinea. J Oceanogr 48:377–384. https://doi.org/10.1007/BF02234016
Jones K (1990) Nitrogen fixation by Lyngbya sp., a marine tropical cyanobacterium. Br Phycol Soc 25:287–289
Kana TM, Weiss DL (2004) Comment on" Comparison of Isotope Pairing and N2: Ar Methods for Measuring Sediment Denitrification" by BD Eyre, S Rysgaard, T Dalsgaard, P Bondo Christensen. 2002. Estuaries 27(1):173–176
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwel JC (1994) Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66(23):4166–4170
Kennish MJ, Bricker SB, Dennison WC, Glibert P, Livingston RJ et al (2007) Barnegat Bay—Little Egg Harbor estuary: case study of a highly eutrophic coastal bay system. Ecol Appl 17(5):S3–S16
Knapp AN (2012) The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front Microbiol 3:1–14
Latimer JS, Charpentier MA (2010) Nitrogen inputs to seventy-four southern New England estuaries: application of a watershed nitrogen loading model. Estuar Coast Shelf Sci 89:125–136
Lipschultz F, Cunningham JJ, Stevenson JC (1979) Nitrogen fixation associated with four species of submerged angiosperms in the central Chesapeake Bay. Estuar Coast Mar Sci 9:813–818. https://doi.org/10.1016/S0302-3524(79)80014-6
Marcarelli AM, Wurtsbaugh WA (2006) Temperature and nutrient supply interact to control nitrogen fixation in oligotrophic streams: an experimental examination. Limnol Oceanogr 51(5):2278–2289
Marcarelli AM, Fulweiler RW, Scott JT (2022) Nitrogen fixation: a poorly understood process along the freshwater-marine continuum. Limnol Oceanogr Lett 7:1–10. https://doi.org/10.1002/lol2.10220
Marino R (2001) An experimental study of the role of phosphorus, molybdenum, and grazing as interacting controls on planktonic nitrogen fixation in estuaries. Ph.D. dissertation. Cornell University.
McGlathery KJ (2008) Seagrass habitats. In: Capone DG, Bronk DA, Mulholland MR, Carpenter E (eds) Nitrogen the marine environment, 2nd edn. Elsevier, New York, pp 1037–1071
McGlathery KJ, Sundback K, Anderson IC (2007) Eutrophication in shallow coastal bays and lagoons: the role of plants in the coastal filter. Mar Ecol Prog Ser 348:1–18
McRoy CP, Goering JJ, Chaney B (1973) Nitrogen fixation associated with seagrasses. Limnol Oceanogr 18(6):998–1002
Montoya JP, Voss M, Kähler P, Capone DG (1996) A simple, high precision, high-sensitivity tracer assay for N2 fixation. Appl Environ Microbiol 62(3):986–993
Moriaty DJW, O’Donahue MJ (1993) Nitrogen fixation in seagrass communities during summer in the Gulf of Carpentaria, Australia. Aust J Mar Freshwater Res 44:117–125
Nichols L (2017) Propagation of error, from organic chemistry laboratory techniques. https://chem.libretexts.org/@go/page/353. Accessed 29 June 2021
Nixon SW, Buckley B, Granger S, Bintz J (2001) Responses of very shallow marine ecosystems to nutrient enrichment. Hum Ecol Risk Assess 7:1457–1481
O’Donohue MJ, Moriarty DJW, Mac Rae IC (1991) Nitrogen fixation in sediments and the rhizosphere of the seagrass Zostera capricorni. Microb Ecol 22(1):53–64
Patriquin D, Knowles R (1972) Nitrogen fixation in the rhizosphere of marine angiosperms. Mar Biol 16:49–58
Ptacnik R, Anderson T, Tamminen T (2010) Performance of the Redfield ratio and a family of nutrient limitation indicators as thresholds for phytoplankton N vs. P Limitation. Ecosystems 13:1201–1214
Reynolds LK, Marino R, Muth MF, McLenaghan N, Hayn M, Tyler AC, McGlathery KJ, Howarth RW (2015) Evidence of grazer control on nitrogen fixation by eelgrass epiphytes in a temperate coastal bay. Mar Ecol Prog Ser 526:11–19. https://doi.org/10.3354/meps11234
Stewart WDP, Fitzgerald GP, Burris RH (1967) In-situ studies on N2 fixation, using the acetylene reduction technique. Proc Natl Acad Sci USA 58:2071
Valiela I, Foreman K, LaMontagne M, Hersh D et al (1992) Couplings of watersheds and coastal waters: sources and consequences of nutrient enrichment in Waquoit Bay Massachusetts. Estuaries 15:443–457
Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57:1–45. https://doi.org/10.1023/A:1015798428743
Wazniak CE, Hall MR, Carruthers TJB, Sturgis B, Dennison WC, Orth RJ (2007) Linking water quality to living resources in a mid-Atlantic lagoon system, USA. Ecol Appl 17(5):S64–S78
Welsh DT (2000) Nitrogen fixation in seagrass meadows: Regulation, plant-bacteria interactions and significance to primary productivity. Ecol Lett 3:58–71
Wilson ST, Böttjer D, Church MJ, Karl DM (2012) Comparative assessment of nitrogen fixation methodologies conducted in the oligotrophic North Pacific. Appl Environ Microbiol 78:6516–6523
Acknowledgements
We thank Ken Foreman for leadership contributions to the design and implementation of the coupled biogeochemical cycles study (2005-2009) that the long-term data collection here began under, Jane Tucker for the MIMS analysis, Marshall Otter for the N isotope analysis, Katie Haviland, Meredith Ferdie, Laura Reynolds, Kim Holzer, Audrey Vinton, Mary Gibbs, Eli Perrone, and Sam Kelsey for assistance in the field and/or laboratory.
Funding
This work was supported by NSF grant 0420575 from the Biocomplexity Program from 2004 to 2010, two awards from Woods Hole SeaGrant from 2010 to 2016, NSF grants 1654845 and 231106 from the LTREB Program from 2016 to 2028, and an endowment to Cornell University (in support of RWH) from David R. Atkinson.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MH, RM, AEG (15N and N2:Ar experiments), and KJM (2005–2010). The first draft of the manuscript was co-written by RWH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Thad Scott.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marino, R., Hayn, M., Howarth, R.W. et al. Nitrogen fixation associated with epiphytes on the seagrass Zostera marina in a temperate lagoon with moderate to high nitrogen loads. Biogeochemistry 166, 211–226 (2023). https://doi.org/10.1007/s10533-023-01083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01083-2