Comprehensive Evaluation of Psychotic Features and Their Clinical Correlates in Early Parkinson’s Disease
Abstract
Background:
Some reports suggest that psychotic features may occur in the early stages of Parkinson’s disease (PD), but sensitive tools have not been utilized.
Objective:
The aim was to evaluate the presence of psychotic symptoms using detailed scales and to assess the association with clinical characteristics.
Methods:
Healthy controls and patients within three years of PD onset were recruited. Participants were examined for psychotic symptoms using two different instruments: the Comprehensive Assessment of At-Risk Mental States (CAARMS) and a 10 question PD specific psychosis severity scale (10PDQ). In the PD group, medication use, motor and non-motor symptoms were documented.
Results:
Based on CAARMS and 10PDQ scales, psychotic features were present in 39% (27/70) of patients and 4% (3/74) of controls. The prevalence of passage hallucinations and illusions was significantly higher in PD compared to the control group. The presence of PD-associated psychotic features was not significantly affected by medication, motor severity or global cognitive status. Higher prevalence of overall non-motor manifestations, REM sleep behavior disorder (RBD) and depressive symptoms was significantly associated with the manifestation of psychotic features in PD [(adjusted OR:1.3; 95% CI:1.1-1.6; p = 0.003), (adjusted OR:1.3; 95% CI:1.0-1.6; p = 0.023), and (adjusted OR:1.2; 95% CI:1.0-1.4;p = 0.026)].
Conclusions:
Psychotic phenomena mainly of minor nature are highly common in early PD. Cumulative non-motor symptoms, RBD and depressive features are associated with the presence of psychotic symptoms in this non-demented, early-stage PD population. More studies are needed to clarify the mechanisms that contribute to the onset of psychotic features in early PD.
INTRODUCTION
Parkinson’s disease (PD)-associated psychosis is one of the most debilitating non-motor features and has been linked to multiple adverse outcomes. Ravina et al. (2007) proposed that the presence of illusions, false sense of presence, hallucinations or delusions occurring after the onset of PD, with a duration of one month that could not be attributed to another cause of parkinsonism or primary psychiatric disorder summarize the key components of the entity of psychosis in PD [1]. Since then, visual and auditory hallucinations in PD have been identified as the most prevalent manifestations of PD-psychosis in advanced stages of PD [2]. In contrast, hallucinations of other sensory modalities, minor hallucinations (presence hallucinations, passage hallucinations, and illusions) and delusional ideas have been relatively understudied. Minor psychotic phenomena have recently gained more attention, as they have been reported to be the most common type of hallucination in early stages of PD, even predating the onset of motor symptoms [3].
Several cognitive, non-motor and motor factors have been proposed to relate to psychotic features in moderate and late stages of PD. However, the clinical comorbidities that could be implicated in the exhibition of psychotic manifestations in earlier stages of PD are still under investigation [4].
The impact of, even minor, psychotic phenomena on the course of the disease is substantial, as they can lead to decreased quality of life and significant patient and caregiver distress. Therefore, the need of an early screening of psychotic symptoms becomes crucial [5]. The main objective of the current study was to evaluate a wide spectrum of psychotic symptoms in the early PD population, by using detailed psychiatric measures, and to identify clinical features that contribute to their occurrence.
MATERIALS AND METHODS
Participants
The study had a cross-sectional design. The study population was recruited from two-centers, the Movement Disorder Clinics in the 1st Neurology Department of National and Kapodistrian University of Athens (NKUA) in Aiginiteion Hospital and the 2nd Neurology Department of NKUA in Attikon University Hospital. The Movement Disorder Society (MDS) Clinical Diagnostic Criteria for PD were implemented to support the diagnosis in patients of the sample [6]. Patients within 3 years of symptom onset were included, since the focus of the study was to probe into the early stage of PD, and to eliminate potential disease or medication-related confounders that occur with disease progression. Individuals with no symptoms of PD or dementia and no family history of PD were grouped as “healthy controls” and were mainly age-matched spouses of patients with PD.
Clinical assessments
Demographic characteristics were documented for all individuals. Data on education level and clinical characteristics such as family history of PD, duration of disease and age of symptom onset were recorded for the PD group. Information on treatment strategies including levodopa equivalent daily dose (LEDD) and use of specific drug categories (levodopa, dopaminergic agonists, and MAO inhibitors) was also documented. The method for converting the total daily dopaminergic therapeutic dose in LEDD was obtained from published formulas [7].
Motor signs were evaluated using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part II and III motor subscales [8] and the Hoehn and Yahr (HY) scale [9]. Individuals’ ability to function in activities of daily living was assessed by the Schwab and England (SE-ADL) Activities of Daily Living scale [10]. Patients were classified as having tremor-dominant (i.e., presenting marked resting tremor with mild bradykinesia or rigidity), akinetic-rigid (i.e., presenting marked akinesia or bradykinesia and rigidity with no or only mild tremor) or mixed phenotypes, depending on their essential motor manifestation. Laterality of motor symptoms (right or left dominant or symmetrical) was also documented.
Further, non-motor features were measured using the MDS-UPDRS part I scale [8]. Other measures included the REM sleep behavior disorder screening questionnaire (RBDQ), with a screening cutoff of≥6 indicating probable RBD since no video polysomnography was performed [11], and the Sniffin’ Sticks Screening test assessing olfaction [12]. Global cognitive abilities were assessed with the Montreal Cognitive Assessment (MoCA) and mild cognitive impairment for patients with PD was defined at the recommended cutoff value of < 26 [13]. Frontal Assessment Battery (FAB) was implemented to examine the executive and frontal lobe dysfunction in patients with PD [14]. Depression was examined using the 15-item Geriatric Depression Scale (GDS) with a cutoff score of≥5 indicating presence of clinically significant depressive features [15]. The Questionnaire for Impulsive-Compulsive Disorders in PD-Rating Scale (QUIP-RS) was performed to examine Impulse Control Disorders (ICDs) [including compulsive gambling, buying, sexual behavior and eating] and related disorders (including hobbying, punding, and dopamine dysregulation syndrome, DDS) and the optimal cutoff point for combined ICDs was≥10 [16].
Evaluation of psychotic features
Trained clinicians (IP, VP) assessed patients for psychotic symptoms using two different instruments: an easy-to-administer 10 question PD specific psychosis severity scale (10PDQ) [17] and a semi-structured evaluation tool used to identify individuals at ultra-high risk of developing psychosis, the Comprehensive Assessment of At-Risk Mental States (CAARMS) [18].
The 10PDQ scale contains ten items. The first five questions identify the type of hallucination (visual, auditory, olfactory, sense of presence) or delusion, while the last five quantify the intensity, frequency, insight, and impact of the worst psychotic experience in the life of the patient and the family. The range of score for each item is 0-4 and the total score adds all ten items (range: 0-40). Subjects were defined as “10PDQ cases” when they had a total score > 0 [17].
The CAARMS is designed to assess psychopathology and to determine if an individual meets criteria for being at an ultra-high risk state of developing a first-episode psychotic disorder. Details on the rationale and the rating components of CAARMS have been previously presented in the literature [18]. Since the main interest of the study was to examine the psychotic symptoms in early PD, the abbreviated version of CAARMS and only the positive symptom domain of full CAARMS, including unusual thought content (CAARMS 1.1), non-bizarre ideas (CAARMS 1.2), perceptual abnormalities (CAARMS 1.3), and disorganized speech (CAARMS 1.4), was used. Each of the four categories of the positive symptom domain has subscales that measure symptom frequency and duration, pattern of symptoms and level of distress and a rating scale that measures symptom intensity (Global Rating Scale). For the purpose of the current study, CAARMS was used to assess a plethora of psychotic symptoms, yet not to identify individuals at ultra-high risk of psychosis [18].
Using an inclusive approach, participants were considered to be experiencing psychotic features in case they had a score > 0 in 10PDQ scale or a score > 1 in one of the four categories of positive symptom domain in CAARMS scale. Subjects with a score of 0 in the 10PDQ scale or a score≤1 in each sub-category of the positive symptom assessment in CAARMS scale were considered as non-psychotic. Subjects presenting visual and auditory disturbance due to primary conditions such as visual or hearing loss, were not grouped along with individuals with psychotic manifestations.
Using the same criteria for defining psychotic features, the PD group was divided into two sub-groups:the PD-psychotic (PDP) and PD-non-psychotic (PDnP) group.
Ethics
The study protocol was approved by the institutional Ethics Committees of the Neurology Departments and conducted in accordance with the latest version of the Declaration of Helsinki. All participants provided written informed consent before study participation.
Statistical analysis
Statistical analyses were performed using the statistical software programs IBM SPSS, version 25.0 (USA). Categorical variables were summarized as absolute numbers and percentages. Continuous variables were presented as medians (Q1, Q3) or mean±statistical deviation (SD). Total score in the 10PDQ scale and individual scores in four subcategories of CAARMS scale were treated as continuous variables. The presence of any psychotic feature was computed as categorical variable.
In the primary analysis, psychotic manifestations, treated either as continuous or categorical variables, and demographic characteristics (age and sex) were compared between patients with PD and healthy individuals using the chi-square (χ2) test, Mann-Whitney non-parametric test and Student t test, as appropriate. Generalized linear models (GLMs) were computed in order to evaluate the association between 10PDQ and CAARMS scores and clinical variables such as age, sex, and diagnosis of PD.
Subsequently, bivariate comparisons between PDP and PDnP subjects were performed using a two-sample t test or non-parametric Mann-Whitney test (for continuous variables) and the chi-square test (for categorical variables). Variables associated with psychosis at p < 0.10 on bivariate analysis were included in multivariate analyses using logistic regression models. Binary logistic regression was used to derive the odds ratio (OR) for demographics (age and sex), motor and non-motor features between PDP and PDnP groups. Adjusted for age, OR were then computed with binary logistic regression with hierarchical entry method. The level of statistical significance was set at p≤0.05.
An explanatory analysis was performed to compare the use of a brief screening tool for psychosis in PD, the 10PDQ scale, to a detailed psychopathology interview, the CAARMS. The association strength between 10PDQ scores and CAARMS global rating scores was examined using the Spearman’s rank correlation coefficient and these results are represented in the Supplementary Material.
RESULTS
Subject characteristics
A total of 144 participants were included in the sample. 70 subjects were diagnosed with PD and had a mean age of 63 years (range: 32-86 years) and 74 individuals were grouped as healthy controls with a mean age of 68 years (range: 50-89) [p = 0.050]. 67% were male in the PD group, compared to 37% in healthy individuals [χ2 = 13.5, df = 1, p < 0.001].
Table 1 illustrates the comparisons of psychotic features treated either as continuous or categorical variables between patients and controls. Total scores in 10PDQ scale were significantly higher in patients with PD, compared to controls, even after adjusting for age and sex (p < 0.001).
Table 1
Correlates of psychotic features between HC and PD groups
| PD patients N = 70 | Healthy controls N = 74 | adjustedp | ||
| Age, y | 63 ± 11.9 | 68 ± 7.9 | 0.050 | |
| [mean ± SD] | ||||
| Male Sex | 47 [67] | 27 [37] | <0.001 | |
| [N,% ] | ||||
| 10PDQ (total score) | 2.6 ± 4.4 | 0.1 ± 0.8 | <0.001 | |
| [mean ± SD] | ||||
| 10PDQ | 23 [33] | 2 [3] | <0.001 | |
| [cases,% ] | ||||
| CAARMS 1.1 section | Unusual Thought Content-Global Rating Scale | 0.1±0.3 | 0.0±0.0 | 0.124 |
| [mean±SD] | ||||
| CAARMS 1.1 | Unusual Thought Content | 2 [3] | 0 [0] | 0.235 |
| section | [cases,% ] | |||
| CAARMS 1.1 | Frequency and Duration | 0.1±0.5 | 0.0±0.0 | 0.109 |
| section | [mean±SD] | |||
| CAARMS 1.2 | Non-bizarre Ideas-Global Rating Scale | 0.2 ± 0.6 | 0.0 ± 0.0 | 0.031 |
| section | [mean ± SD] | |||
| CAARMS 1.2 section | Non-bizarre Ideas | 4 [6] | 0 [0] | 0.053 |
| [cases,% ] | ||||
| CAARMS 1.2 section | Frequency and duration [mean ± SD] | 0.2 ± 1.0 | 0.0 ± 0.0 | 0.028 |
| CAARMS 1.3 section | Perceptual Abnormalities-Global Rating Scale | 0.8 ± 1.4 | 0.1 ± 0.5 | <0.001 |
| [mean ± SD] | ||||
| CAARMS 1.3 section | Perceptual Abnormalities | 23 [33] | 4 [5] | <0.001 |
| [cases,% ] | ||||
| CAARMS 1.3 section | Frequency and duration [mean ± SD] | 0.6 ± 1.1 | 0.1 ± 0.2 | <0.001 |
| CAARMS 1.4 section | Disorganized Speech-Global Rating Scale | 0.5 ± 1.0 | 0.0 ± 0.0 | <0.001 |
| [mean ± SD] | ||||
| CAARMS 1.4 section | Disorganized Speech | 13 [19] | 0 [0] | <0.001 |
| [cases,% ] | ||||
| CAARMS 1.4 section | Frequency and Duration | 0.4 ± 1.0 | 0.0 ± 0.0 | <0.001 |
| [mean ± SD] |
Data are given as mean±SD or N (%). Significance level for comparison is p < 0.05. 10PDQ, 10 question PD specific psychosis severity scale; CAARMS, Comprehensive Assessment of At-Risk Mental States; HC, healthy controls; PD, Parkinson’s disease; SD, standard deviation. Each of the four categories of the positive symptom domain (CAARMS 1.1, 1.2, 1.3, and 1.4) has subscales that measure symptom frequency and duration, pattern of symptoms and level of distress and a rating scale that measures symptom intensity (Global Rating Scale). The Global Rating Scale rates symptom severity from 0 (absent psychotic feature) to 6 (severe psychotic feature). The frequency and duration subscales are rated using a 0 (absent psychotic symptom) to 6 (continuous psychotic symptom) rating scale. The pattern of symptoms addresses whether and to what extent substance use is related to presence of psychotic features examined (0: no relation, 1: psychotic feature occurs in relation to substance use and at other times as well, 2: noted only in relation to substance use). The level of distress scale is a 100-point Likert scale (0: not distressed at all, 100: extremely distressed) that subjectively measures the distress related to each subscale.
The global severity and frequency scores of the unusual thought content in CAARMS (CAARMS 1.1 section) were found relatively low in both patients and controls (p = 0.124 and p = 0.109 respectively). The severity and frequency scores of non-bizarre ideation (CAARMS 1.2 section) were significantly higher in PD patients, compared to healthy individuals. The absolute number of participants defined as cases with delusional ideation was higher in patients, but this difference was not significant (χ2 = 4.3, df = 1, p = 0.053).
The majority of patients and controls have experienced mild perceptual abnormalities (patients with PD: range = 0-6, healthy individuals: range = 0-2), as scores in CAARMS 1.3 section indicate. Regarding frequency, the distribution in PD patients was heavily skewed with a large proportion of “once a month to twice a week - less than one hour per occasion” responses in the 1.3 frequency subscale of CAARMS. These differences are illustrated in the boxplots 2 and 1 respectively (Supplementary Material). Following clinical covariate adjustment, higher prevalence of perceptual abnormalities was noticed in patients with PD compared to controls: 33% (23 out of 70) of patients and 5% (4 out of 74) of healthy individuals were defined as cases based on the CAARMS interview [χ2 = 17.8, df = 1, p < 0.001].
All healthy individuals reported normal organized speech. In patients with PD, the range of severity and frequency scores in the disorganized speech section (CAARMS 1.4) were 0-3 and 0-4 respectively. However, the majority of patients classified as cases with disorganized speech (8 out of 13, 62%) reported slight subjective difficulties with a frequency of “once a month to twice a week-less than one hour per occasion”.
Assessment of psychotic features comparing 10PDQ scale to CAARMS interview
Based on the 10PDQ scale, 33% (23/70) of PD patients were identified as cases by the 10PDQ, compared to 3% (2/74) of healthy controls (χ2 = 22.8, df = 1, p < 0.001). 39% (27/70) of patients with PD have reported any kind of psychotic feature using CAARMS interview, compared to 4% (3/74) of healthy individuals (χ2 = 26.0, df = 1, p < 0.001). All subjects identified as cases using the 10PDQ scale were included in the group presenting psychotic features according to CAARMS interview regarding both PD and healthy categories.
There was a slight discrepancy in identifying psychotic manifestations using 10PDQ and CAARMS instruments. In the PD group, four patients have reported psychotic features that were identified by CAARMS, but not using 10PDQ scale. These symptoms mainly involved delusional ideation of guilt, grandiosity and less common forms of hallucinations (i.e., tactile). In the control group, one individual reported a delusional ideation of guilt, which was identified using the CAARMS interview, yet not the10PDQ scale.
Due to these mild differences in the evaluation of psychotic phenomena using the two instruments, the PD group was separated in two sub-categories based on the performance in 10PDQ testing: patients with 10PDQ score > 0 (10PDQ cases) and patients with 10PDQ score = 0 (10PDQ non-cases). Severity and frequency scores of the four categories of positive symptoms in CAARMS interview were compared between 10PDQ cases and non-cases (Supplementary Table 1). Mean scores of the CAARMS subscales did not differ in 10PDQ cases and non-cases, except for the sections of perceptual abnormalities and disorganized speech (severity and frequency scores), which were higher in the cases’ group (p < 0.001, p = 0.001, and p = 0.002). Using the Spearman’s rank correlation coefficient, the 10PDQ total score was significantly correlated with the individual scores of perceptual abnormalities and disorganized speech in CAARMS (Supplementary Table 2).
Spectrum of psychotic symptoms in patients with PD and healthy controls
Based on both CAARMS interview and 10PDQ scale, psychotic features were present in 39% (27 out of 70) of patients with PD and 4% (3 out of 74) of healthy controls [χ2 = 26.0, df = 1, p < 0.001].
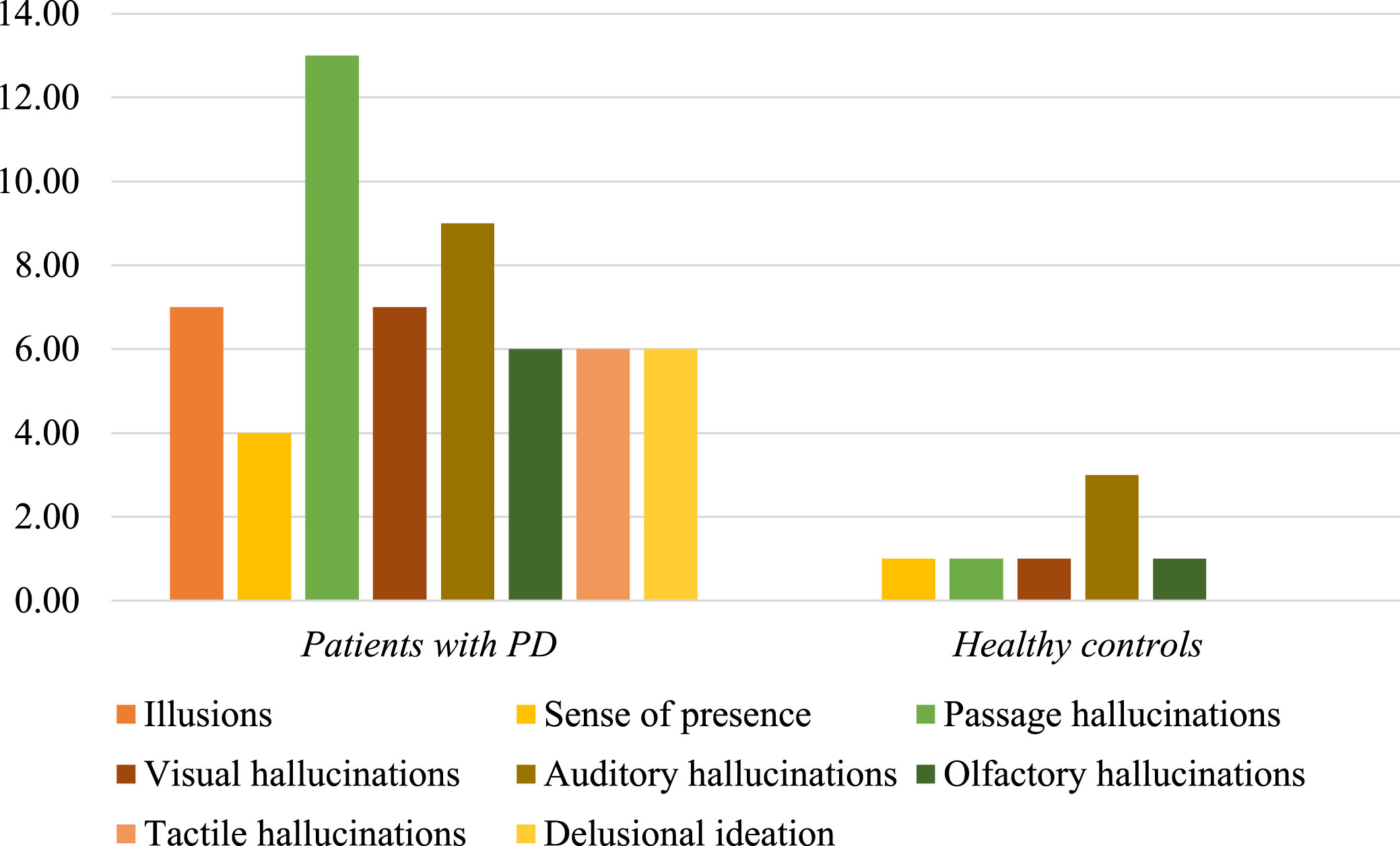
Information about modality and quality of psychotic manifestations was retrieved from the individual items of both 10PDQ and CAARMS scales. In healthy controls, auditory disturbances were more frequent followed by minor psychotic phenomena, visual and olfactory hallucinations. Illusions, tactile hallucinations, and delusional ideation were not observed in controls.
In the PD group, there was a predominance of passage hallucinations followed by auditory phenomena, illusions and formed visual hallucinations, olfactory and tactile abnormalities, delusional ideation and, finally, sense of presence (Fig. 1). Among all modalities of psychotic symptoms, the presence of minor hallucinations including passage hallucinations and illusions was significantly higher in patients with PD compared to healthy individuals, even after controlling for age and sex between the two study groups (χ2 = 7.4, df = 1, p = 0.008 and χ2 = 5.5, df = 1, p = 0.025, respectively, Supplementary Material).
Fig. 1
Psychotic features in patients with PD and healthy controls. The bar chart illustrates the distribution of several modalities of psychotic manifestations in healthy controls and patients with PD. In healthy subjects, auditory disturbances were more frequent followed by minor psychotic phenomena, visual and olfactory hallucinations. In the PD group, there was a predominance of passage hallucinations followed by auditory phenomena, illusions and formed visual hallucinations, olfactory and tactile abnormalities, delusional ideation and, finally, sense of presence. The presence of passage hallucinations and illusions was significantly higher in patients with PD, compared to healthy individuals.
Clinical factors associated with psychosis in PD
The prevalence of psychotic features in the PD sample was 39% (27 out of 70). No individual received antipsychotic medication at the time of evaluation. Subjects with psychotic features (PDP group, n = 27) were older compared to patients without such features (PDnP group, n = 43) (p = 0.026). No difference in sex or education level was detected between the two groups [(χ2 = 2.7, df = 1, p = 0.185), p = 0.083] (Table 2).
Table 2
Clinical and demographic characteristics in PDP-PDnP groups
| PDP N = 27 | PDnP N = 43 | adjusted p | |
| Demographics | |||
| Age, y | 71 (6 2 ,75) | 6 2 (5 2 ,7 0) | 0.026 |
| Median (Q1,Q3) | |||
| Male Sex | 15 (56) | 32 (74) | 0.185 |
| N, % | |||
| Education, y | 15 (9,16) | 15 (12,17) | 0.081 |
| Median (Q1,Q3) | |||
| PD-associated clinical features | |||
| Family History | 9 (36) | 11 (26) | 0.421 |
| N, % | |||
| Age of Onset, y | 69 (59,73) | 61 (50,69) | 0.022 |
| Median (Q1,Q3) | |||
| Duration, y | 2 (1,2) | 2 (1,2) | 0.362 |
| MDS-UPDRS III | 21 (17,27) | 22 (16,25) | 0.522 |
| Median (Q1,Q3) | |||
| HY | 2 (2,2) | 2 (1,2) | 0.055 |
| Median (Q1,Q3) | |||
| SE-ADL | 90 (90,100) | 100 (90,100) | 0.131 |
| Median (Q1,Q3) | |||
| Akinetic Type | 3 (12) | 6 (14) | 0.342 |
| N, % | |||
| Left Laterality | 11 (48) | 15 (48) | 0.662 |
| N, % | |||
| Medication | |||
| LEDD, mg/day | 300 (160,400) | 150 (0,360) | 0.083 |
| Median (Q1,Q3) | |||
| L-Dopa use | 16 (59) | 20 (47) | 0.335 |
| N, % | |||
| Dopamine agonist use | 12 (44) | 19 (44) | 1.000 |
| N, % | |||
| MAOI | 8 (30) | 7 (16) | 0.236 |
| N, % | |||
| Non-motor Features | |||
| MDS-UPDRS part I* | 7 (5,11) | 5 (3,6) | 0.034 |
| Median (Q1,Q3) | |||
| MDS-UPDRS part II | 7 (3,10) | 5 (2,8) | 0.171 |
| Median (Q1,Q3) | |||
| RBDQ | 6 (3,7) | 3 (2,5) | 0.046 |
| Median (Q1,Q3) | |||
| Sniffin’ Sticks | 6 (4,9) | 7 (6,8) | 0.883 |
| Median (Q1,Q3) | |||
| QUIP-RS | 0 (0,1) | 0 (0,0) | 0.123 |
| Median (Q1,Q3) | |||
| MOCA | 25 (23,28) | 27 (24,29) | 0.141 |
| Median (Q1,Q3) | |||
| FAB | 16 (14,17) | 17 (16,18) | 0.0 4 2 |
| Median (Q1,Q3) | |||
| GDS | 4 (1,7) | 1 (0,3) | 0.0 31 |
| Median (Q1,Q3) |
Data are given as median (Q1, Q3) or N (%). Significance level for comparison is p < 0.05. PDP: individuals with PD-associated psychosis, PDnP, patients with PD without psychosis; LEDD, Levodopa equivalent daily dose; MDS-UPDRS, Movement-Disorder-Society Unified Parkinson’s Disease Rating Scale; RBDQ, REM sleep Behavior Disorder Questionnaire; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; MoCA, Montreal Cognitive Assessment; FAB, Frontal Assessment Battery; GDS, Geriatric Depression Scale.
PDP individuals were older at disease onset, compared to PDnP subjects (p = 0.022). The median duration of PD was approximately two years in both groups (range = 0.5-3 years, p = 0.362). Regarding motor assessment, performance in MDS-UPDRS part II, III, HY and SE-ADL scales was similar in the two groups (Table 2). PDP and PDnP individuals did not present differences in type of PD (χ2 = 0.7, df = 2, p = 0.342) or in laterality of parkinsonian symptoms (χ2 = 1.6, df = 2, p = 0.662).
11 out of 70 patients with PD (PDP group: n = 2 [7% ] and PDnP group: n = 9 [21% ]) did not receive any treatment at the time of evaluation. 10% (7 out of 70) of PD patients were under anticholinergic treatment. Total LEDD was higher in the PDP group, compared to PDnP individuals, yet this difference was not statistically significant (p = 0.083). There was no difference in the prescription of a specific drug category (l-dopa, dopamine agonists or MAO inhibitors) between the two groups (p = 0.335, p = 1.000, p = 0.236, respectively). In terms of other treatment strategies, no individual received medication for cognitive impairment, while 16% (11 out of 70) of patients with PD were under antidepressant treatment, of which the majority (91%, 10/11) received selective serotonin reuptake inhibitors.
Higher scores in MDS-UPDRS part I scale (after excluding the item of hallucinations and psychosis), RBDQ scale, FAB scale, and GDS scale were noted in the PDP group, compared to the PDnP individuals after adjustment for age (p = 0.034, p = 0.046, p = 0.046, p = 0.031, respectively). Even though the mean total MoCA score was similar between the two groups, there was a trend for visuospatial, executive, naming and abstraction deficits in PDP patients (Supplementary Table 4). In the binary regression models, higher MDS-UPDRS part I score, RBDQ scores and GDS scores demonstrated a trend association with psychosis, even after adjustment for clinical covariates. FAB marginally failed to be significantly associated in these models(Table 3).
Table 3
Logistic Regression Model examining non-motor features in PDP and PDnP groups
| PDP N = 27 | PDnP N = 43 | Unadjusted β | Adjusted β | |
| [95% CI;p-value] | [95% CI;p-value] | |||
| MDS-UPDRS part I | 7 (5,11) | 5 (3,6) | 1.3 [1.1-1.6; 0.001] | 1.3 [1.1-1.6; 0.003] |
| Median (Q1,Q3) | ||||
| MDS-UPDRS part II | 7 (3,10) | 5 (2,8) | 1.1 [1.0-1.2; 0.202] | 1.1 [0.9-1.2; 0.321] |
| Median (Q1,Q3) | ||||
| RBDQ | 6 (3,7) | 3 (2,5) | 1.3 [1.1-1.6; 0.006] | 1.3 [1.0-1.6; 0.023] |
| Median (Q1,Q3) | ||||
| Sniffin’ Sticks | 6 (4,9) | 7 (6,8) | 1.0 [0.8-1.2; 0.868] | 1.1 [0.9-1.3; 0.575] |
| Median (Q1,Q3) | ||||
| QUIP-RS | 0 (0,1) | 0 (0,0) | 1.1 [0.9-1.2; 0.325] | 1.1 [0.9-1.2; 0.479] |
| Median (Q1,Q3) | ||||
| MoCA | 25 (23,28) | 27 (24,29) | 0.9 [0.7-1.0; 0.145] | 0.9 [0.8-1.2; 0.586] |
| Median (Q1,Q3) | ||||
| FAB | 16 (14,17) | 17 (16,18) | 0.8 [0.7-1.0; 0.046] | 0.9 [0.7-1.1; 0.240] |
| Median (Q1,Q3) | ||||
| GDS | 4 (1,7) | 1 (0,3) | 1.2 [1.1-1.5; 0.010] | 1.2 [1.0-1.4; 0.026] |
| Median (Q1,Q3) |
Data are given as median (Q1, Q3) or N (%). Significance level for comparison is p < 0.05. PDP, individuals with PD-associated psychosis; PDnP, patients with PD without psychosis; LEDD, Levodopa equivalent daily dose; MDS-UPDRS, Movement-Disorder-Society Unified Parkinson’s Disease Rating Scale; RBDQ, REM sleep Behavior Disorder Questionnaire; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; MoCA, Montreal Cognitive Assessment; FAB, Frontal Assessment Battery; GDS, Geriatric Depression Scale.
DISCUSSION
The main objective of this study was to evaluate the prevalence of psychotic features in early PD using detailed measures. More than one third of patients with early PD reported at least one type of psychotic symptom, compared to healthy individuals. The prevalence of passage hallucinations and illusions reached a significant difference between controls and PD patients. Furthermore, motor examination and PD medication did not differ between patients with and without early-onset PD psychotic features. The presence of non-motor symptoms, RBD and depressive features seem to mildly increase the probability of exhibiting PD-associated psychotic manifestations. Frontal dysfunction marginally did not significantly correlate to psychotic features in this early PD population.
The novelty of our study was that a detailed psychiatric analysis by two separate scales was used to identify psychotic features in early PD. On the one hand, the implementation of CAARMS interview enabled clinicians to comprehensively probe into the nature of psychotic phenomena, given the open-ended structure of the questions that require further elaboration on examinees’ responses regarding severity and the frequency. Ambiguous answers were excluded, according to CAARMS global rating scale, in order to identify only frank psychotic phenomena. Moreover, CAARMS was the first instrument designed to identify imminent development of first-episode psychotic disorder, through an extensive exploration of subclinical psychopathology [18]. It maps, thus, the psychopathological symptoms of subclinical intensity, below the sensitivity threshold of other clinical scales, such as Brief Psychiatric Rating Scale (BPRS) and the Positive and Negative Symptom Scale (PANSS) that have been used in other PD clinical studies [19, 20]. On the other hand, 10PDQ scale is specifically designed for PD-associated psychosis and has been developed based on previous non-validated versions that have been implemented in clinical trials. [21, 22]. It explores the severity of the most common psychotic symptoms in PD, including sense of presence, visual, auditory, and olfactory hallucinations and delusional ideation of infidelity or persecution, in a 4-point scale with an excellent inter-rater and significant intra-rater reliability [17]. The worst psychotic experience is evaluated in terms of its duration, insight, affective consequences, and actions. In contradiction to the CAARMS interview, that documents self-distress, 10PDQ scale includes patients’ and family input to account for the poor insight many patients possess. Despite different methodological designs, an association was observed between 10PDQ scores and CAARMS scores addressing perceptual abnormalities (Supplementary Material). This finding implies that psychotic disturbances evaluated by CAARMS interview tend to correspond to the hallucinatory categories of the 10PDQ scale. Therefore, CAARMS assessment may provide a useful instrument for monitoring minor or major psychotic symptoms, especially of hallucinatory nature, in this sample of early PD patients, although its design and psychometric features address first-episode psychotic disorder individuals.
Until present, no “ideal” scale for PD psychosis has been identified [5]. According to the critique of MDS Task Force on Rating Scales in PD, several scales were designed specifically for the PD population, but are still inadequate to capture the entire phenomenology of psychotic features, such as MDS-UPDRS Part I scale [8]. Other psychosis scales derived from psychiatric research, for instance BPRS and PANSS scales, have been implemented, although several items relevant to schizophrenia are less useful to PD psychosis [19, 20]. Besides, these tools could be cumbersome for everyday clinical practice, due to their complex form. There is still lack of a valid questionnaire addressing psychotic manifestations in early PD and emphasizing minor hallucinations. Our attempt to combine an open-ended interview with another scale better at cataloging specific features, enabled us to identify a significantly high prevalence of psychotic features in the early period of PD.
One of the primary outcomes of our study was that perceptual abnormalities, especially minor, were dominant in the early PD population. This finding is in accordance with recent literature [23]. Early reports in the pre-levodopa period have described patients with illusions and visual hallucinations [24], even in the absence of prominent cognitive impairment. Several cohorts of early PD population, such as PRIAMO and PPMI studies, have observed an increasing prevalence of psychotic features over a 2-year period from 3% to 10% [25, 26]. In another study of “honeymoon” period of PD, Erro et al. (2016) identified a significant rise of prevalence of psychotic symptoms over a 4-year evaluation from 1.4% to 13.5% for hallucinations and 12.2% for delusions [27]. In our series, psychotic symptoms were present in 39% of patients with PD. According to literature, the highest prevalence of psychotic manifestations during the pre-levodopa era was 42%, including mainly minor phenomena in a sample of 50 drug-naïve PD patients [3]. Among these studies, there is a considerable discrepancy in point-prevalence of PD-associated psychosis due to methodological issues. Yet, all these data emphasize that psychotic symptoms can manifest early in the course of the disease.
A range of possible motor and non-motor factors that could be related to psychotic experiences was investigated in our PD sample. Patients with psychotic symptoms were older and had an older age of symptom onset compared to individuals without psychosis. Moreover, consistent with previous findings, performance in several motor measurements was similar between psychotic and non-psychotic early PD patients [3, 28–30]. It has been previously reported that visual hallucinations are observed in patients with greater motor severity of disease, even though this was generally in participants with more advanced PD than in our study (mean disease’s duration:1.7 years, HY stage≤2) [4, 31]. Furthermore, no predominance of akinetic-rigid type in patients with psychotic symptoms was detected in our sample. It seems that by splitting PD into tremor dominant or non-tremor dominant (akinetic-rigid or PIGD), the full spectrum of clinical manifestations in PD is not well-captured. Multi-domain classifications including motor, cognitive and psychiatric measures have shown that this classic tremor/PIGD dichotomy has been a less robust and sufficient instrument to predict clinically meaningful outcomes such as mortality, dementia, or PD-associated psychosis [32, 33]. Taken together, severity and type of motor symptoms do not appear to associate with psychotic symptoms, at least at the early period of PD.
There was no significant difference in proportion of participants on dopamine agonist, levodopa, or MAO inhibitors therapy between those with and without psychotic features. Even though individuals presenting psychotic manifestations had higher total LEDD, this difference marginally did not reach statistical significance. In clinical practice, PD psychotic features often emerge in the setting of augmentation of dopaminergic therapy, especially dopamine agonists, and subside with dose reduction or discontinuation [34]; this can occur even in non-demented PD cases, as an “isolated delusional syndrome” [35]. However, PD psychotic features have been recognized even in unmedicated patients [3, 24, 36]. Our findings are in line with contemporary view of the role of medication in PD psychosis, as a “modifier rather than a necessary feature for the diagnosis of PD psychosis”, according to the NINDS-NIMH work group [1].
In our series, the major factors associated with PD psychotic features were non-motor symptoms including RBD. Prior studies have shown that autonomic symptom burden could be an independent risk factor for future hallucinations [37]. In addition, RBD has been widely associated with visual hallucinations and has been characterized as a predictor of future hallucinations [38]. One possible link between RBD and psychotic features in PD might be based on the cholinergic dysfunction observed in both conditions [39]. Reduced cholinergic nucleus 4 (Ch4) density has been recently associated with future psychotic symptoms in early PD subjects [37]. Another possible common basis of RBD and psychotic experiences in PD could be the dysfunction of prefrontal networks. Gan and colleagues (2021) have observed weaker positive couplings between visual network, default mode network (DMN), DMN and basal ganglia network and within DMN in patients with RBD-PD, while functional connectivity changes within the DMN and aberrant connectivity between posterior areas of DMN and visual-processing areas have been reported in patients with PD and minor hallucinations [40, 41]. Finally, reduced grey matter volume in hippocampal and parahippocampal regions, thalamus and limbic regions might be considered as a common substrate in PD patients with RBD and those with visual hallucinations [39].
Furthermore, the role of cognitive impairment in the presence of psychotic features was investigated in our sample. Despite the fact that total mean MoCA scores were relatively similar between patients with and without psychotic manifestations, by analyzing the individual items of MoCA scale, poorer performance in executive, visuospatial, naming and abstraction domains was revealed in patients with PD-associated psychotic symptoms (p < 0.05). This is in accordance with recent literature findings [42]. Visual hallucinations have been associated with multidomain cognitive dysfunction in PD including deficits in executive function [43], sustained attention [44] and visuospatial-perceptual functions [45]. Further, poor frontal lobe function has been identified in some cases of patients with psychosis in early PD [46, 47]. Neuroimaging findings on greater atrophy across frontal and parietal cortices further support our findings [34, 48]. A common idea for determining the role of executive and frontal functions in the emergence of psychotic features in early PD is the disruption in the processing of information across complex attentional networks including dorsal, ventral attentional and DMN networks [49]. Montagnese and colleagues (2023) reported mild language and naming difficulties in patients with visual hallucinations, after detailed examination of a mixture of language-related cognitive domains [50]. The early identification of this characteristic pattern of cognitive impairment in patients with psychotic manifestations, especially in the early stages of the disease, is of great significance to plan prevention therapies in the future and conduct stratified and optimal management of the ongoing neuropsychiatric symptoms.
Regarding other neuropsychiatric symptoms, depressive features mildly increased the probability of expressing psychotic features in our sample. Psychiatric symptoms in the depressive domain have been frequently recorded in PD with psychotic features and have been suggested to predispose to the development of psychotic experiences [4, 25, 51]. These neurocognitive and neuropsychiatric changes could be grouped in a unified “serotoninergic” clinical subtype of psychosis, as Factor and colleagues (2017) have described in a novel classification scheme based on the main neurotransmitter implicated in each patient group (acetylcholine, serotonin, dopamine) [52]. More specifically, this “serotoninergic” type of psychosis refers to psychosis unrelated to global cognitive decline, in which hallucinatory phenomena (most commonly minor hallucinations) are more likely to occur in the context of depression or anxiety. The name of this class of psychosis reveals the underlying pathological mechanisms that bring psychosis and depression in proximity and implicates changes in the serotoninergic systems. Serotonin may contribute to the pathogenesis of complex visuals hallucinations probably via involvement of the serotonin 2 receptor, as clozapine, primavanserin, and other atypical antipsychotics effectively reduce the occurrence of visual abnormalities [53]. Upregulation of the 5HT2A receptors seems to occur in the prefrontal, visual, and temporal cortex areas only in PD patients with psychosis and the resulting enhanced activity at upregulated 5HT2A receptors in the temporal cortex and visual pathways could precipitate the onset of visual hallucinatory experiences [54]. Therefore, depressive and psychotic features in non-demented early PD population could share similar underlying mechanisms of predominantly brainstem and midbrain pathology.
We acknowledge that our study presents some limitations which need to be addressed carefully. Firstly, due to the cross-sectional design of the study, the identification of a temporal and causal relationship between several clinical factors and psychosis could not be precisely evaluated. In addition, a small proportion of the sample might be contaminated by Lewy body dementia cases as hallucinations early in the course of the disease should alert the clinician towards this diagnosis. However, since the diagnosis of PD always preceded the onset of psychotic features in this sample and none of the included patients had dementia at the time of evaluation, this bias should be negligible. Another limitation of the current study is related to the small size of our sample. In particular, patients were grouped together in the PDP group, regardless of whether they had minor type symptoms or more genuine psychotic phenomena. This meant that potential associations with specific symptoms (minor hallucinations/visual hallucinations rather than delusional ideation) and specific predictors could not be examined due to power concerns. Even though the recruitment of both PD patients and controls was based on a two-center basis, the representativeness of the current sample is not ideal. Yet, observer and self-selection bias were eliminated as much as possible. Finally, clinicians were not blind to clinical status (patients-controls), therefore potential expectational bias should be taken into consideration, in terms of magnifying differences in psychotic tendency in patients, compared to controls.
Overall, our study provides evidence that a variety of psychotic features, especially those of minor nature, are common in early PD. A comprehensive psychiatric evaluation including the CAARMS interview and the 10PDQ scale enabled the clinicians to monitor even subtle perceptual abnormalities and delusional ideas. Undoubtedly, a simple, sensitive, and valid screening tool addressing specifically minor phenomena could be part of the “clinical toolbox” in order to identify psychotic symptoms early. Further research in this area could examine whether the presence of early mild psychotic phenomena carries a prognostic significance for the development of more severe psychotic manifestations or possibly cognitive decline later in the disease course.
ACKNOWLEDGMENTS
The authors would like to thank other members of the Outpatient Clinic of Movement disorders, as well as all the patients and their families for their time, assistance, and commitment.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230056.
REFERENCES
[1] | Ravina B , Marder K , Fernandez HH , Friedman JH , McDonald W , Murphy D , Aarsland D , Babcock D , Cummings J , Endicott J , Factor S , Galpern W , Lees A , Marsh L , Stacy M , Gwinn-Hardy K , Voon V , Goetz C ((2007) ) Diagnostic criteria for psychosis in Parkinson’s disease: Report of an NINDS, NIMH work group, Mov Disord 22: , 1061–1068. |
[2] | Chang A , Fox SH ((2016) ) Psychosis in Parkinson’s disease: Epidemiology, pathophysiology, and management, Drugs 76: , 1093–1118. |
[3] | Pagonabarraga J , Martinez-Horta S , Fernández de Bobadilla R , Pérez J , Ribosa-Nogué R , Marín J , Pascual-Sedano B , García C , Gironell A , Kulisevsky J ((2016) ) Minor hallucinationsoccur in drug-naive Parkinson’s disease patients, even from thepremotor phase, Mov Disord 31: , 45–52. |
[4] | Fénelon G , Goetz CG , Karenberg A ((2006) ) Hallucinations inParkinson disease in the prelevodopa era., Neurology 66: , 93–98. |
[5] | Fernandez HH , Aarsland D , Fénelon G , Friedman JH , Marsh L , Tröster AI , Poewe W , Rascol O , Sampaio C , Stebbins GT , Goetz CG ((2008) ) Scales to assess psychosis in Parkinson’s disease: Critiqueand recommendations, Mov Disord 23: , 484–500. |
[6] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease, Mov Disord 30: , 1591–1601. |
[7] | Parkin SG , Gregory RP , Scott R , Bain P , Silburn P , Hall B , Boyle R , Joint C , Aziz TZ ((2002) ) Unilateral and bilateral pallidotomy for idiopathic Parkinson’s disease: A case series of 115 patients, Mov Disord 17: , 682–692. |
[8] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , LaPelle N , Movement Disorder Society UPDRS Revision Task Force ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results, Mov Disord 23: , 2129–2170. |
[9] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism: Onset, progression and mortality, Neurology 17: , 427–42. |
[10] | Schwab RS , England AC (1969) Projection technique for evaluating surgery in Parkinson’s disease. In Third Symposium on Parkinson’s Disease, Billingham FH, Donaldson MC, eds. Churchill Livingstone, Edinburgh, pp. 152-157. |
[11] | Postuma RB , Arnulf I , Hogl B , Iranzo A , Miyamoto T , Dauvilliers Y , Oertel W , Ju YE , Puligheddu M , Jennum P , Pelletier A , Wolfson C , Leu-Semenescu S , Frauscher B , Miyamoto M , Cochen De Cock V , Unger MM , Stiasny-Kolster K , Fantini ML , Montplaisir JY ((2012) ) A single-question screen for REM sleep behavior disorder: A multicenter validation study, Mov Disord 27: , 913–916. |
[12] | Hummel T , Kobal G , Gudziol H , Mackay-Sim A ((2007) ) Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects, Eur Arch Otorhinolaryngol 264: , 237–243. |
[13] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal CognitiveAssessment, MoCA: A brief screening tool for mild cognitiveimpairment, J Am Geriatr Soc 53: , 695–699. |
[14] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB: A Frontal Assessment Battery at bedside, Neurology 55: , 1621–1626. |
[15] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report, J Psychiatr Res 17: , 37–49. |
[16] | Weintraub D , Mamikonyan E , Papay K , Shea JA , Xie SX , Siderowf A ((2012) ) Questionnaire for impulsive-compulsive disorders in Parkinson’s disease–rating scale, Mov Disord 27: , 242–247. |
[17] | Ondo WG , Sarfaraz S , Lee M ((2015) ) A novel scale to assess psychosis in patients with Parkinson’s disease, J Clin Mov Disord 2: , 17. |
[18] | Kollias C , Kontaxakis V , Havaki-Kontaxaki B , Simmons MB , Stefanis N , Papageorgiou C. ((2015) ) Inter-rater reliability of the Greek version of CAARMS among two groups of mental health professionals, Psychiatriki 26: , 217–222. |
[19] | Pollak P , Tison F , Rascol O , Destée A , Péré JJ , Senard JM , Durif F , Bourdeix I ((2004) ) Clozapine in drug induced psychosisin Parkinson’s disease: A randomised, placebo-controlled study withopen follow up, J Neurol Neurosurg Psychiatry 75: , 689–695. |
[20] | Friedman JH , Berman RM , Goetz CG , Factor SA , Ondo WG , Wojcieszek J , Carson WH , Marcus RN ((2006) ) Open-label flexible-dose pilot study to evaluate the safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson’s disease, Mov Disord 21: , 2078–2081. |
[21] | Ondo WG , Levy JK , Vuong KD , Hunter C , Jankovic J ((2002) ) Olanzapine treatment for dopaminergic-induced hallucinations, Mov Disord 17: , 1031–1035. |
[22] | Ondo WG , Tintner R , Voung KD , Lai D , Ringholz G. ((2005) ) Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease, Mov Disord 20: , 958–963. |
[23] | Chendo I , Silva C , Duarte GS , Prada L , Voon V , Ferreira JJ ((2022) ) Frequency and characteristics of psychosis in Parkinson’s disease: A systematic review and meta-analysis, J Parkinsons Dis 12: , 85–94. |
[24] | Aarsland D , Brønnick K , Alves G , Tysnes OB , Pedersen KF , Ehrt U , Larsen JP ((2009) ) The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson’s disease, J Neurol Neurosurg Psychiatry 80: , 928–930. |
[25] | Morgante L , Colosimo C , Antonini A , Marconi R , Meco G , Pederzoli M , Pontieri FE , Cicarelli G , Abbruzzese G , Zappulla S , Ramat S , Manfredi M , Bottacchi E , Abrignani M , Berardelli A , Cozzolino A , Paradiso C , Gaspari CD , Morgante F , Barone P , PRIAMO Study Group ((2012) ) Psychosis associated to Parkinson’s disease in the early stages: Relevance of cognitive decline and depression, J Neurol Neurosurg Psychiatry 83: , 76–82. |
[26] | de la Riva P , Smith K , Xie SS , Weintraub D ((2014) ) Course of psychiatric symptoms and global cognition in early Parkinson disease, Neurology 83: , 1096–1103. |
[27] | Erro R , Picillo M , Vitale C , Amboni M , Moccia M , Santangelo G , Pellecchia MT , Barone P ((2016) ) The non-motor side of the honeymoon period of Parkinson’s disease and its relationship with quality of life: A 4-year longitudinal study, Eur J Neurol 23: , 1673–1679. |
[28] | Zhong M , Gu R , Zhu S , Bai Y , Wu Z , Jiang X , Shen B , Zhu J , Pan Y , Yan J , Zhang L ((2021) ) Prevalence and risk factors for minor hallucinations in patients with Parkinson’s disease, Behav Neurol 2021: , 3469706. |
[29] | Clegg BJ , Duncan GW , Khoo TK , Barker RA , Burn DJ , Yarnall AJ , Lawson RA ((2018) ) Categorising visual hallucinations in early Parkinson’s disease, J Parkinsons Dis 8: , 447–453. |
[30] | Biglan KM Jr , Holloway RG , McDermott MP , Richard IH , Parkinson Study Group CALM-PD Investigators ((2007) ) Risk factors for somnolence, edema, and hallucinations in early Parkinson disease, Neurology 69: , 187–195. |
[31] | Fénelon G , Soulas T , Zenasni F , De Langavant LC ((2010) ) Thechanging face of Parkinson’s disease-associated psychosis: Across-sectional study based on the new NINDS-NIMH criteria, MovDisord 25: , 755–759. |
[32] | Campbell MC , Myers PS , Weigand AJ , Foster ER , Cairns NJ , Jackson JJ , Lessov-Schlaggar CN , Perlmutter JS ((2020) ) Parkinson disease clinicalsubtypes: Key features & clinical milestones, Ann Clin TranslNeurol 7: , 1272–1283. |
[33] | Lawton M , Baig F , Rolinski M , Ruffman C , Nithi K , May MT , Ben-Shlomo Y , Hu MTM ((2015) ) Parkinson’s disease subtypes in the Oxford Parkinson Disease Centre (OPDC) discovery cohort, J Parkinsons Dis 5: , 269–279. |
[34] | Ffytche DH , Creese B , Politis M , Chaudhuri KR , Weintraub D , Ballard C , Aarsland D ((2017) ) The spectrum psychosis in Parkinson disease, Nat Rev Neurol 13: , 81–95. |
[35] | Stefanis N , Bozi M , Christodoulou C , Douzenis A , Gasparinatos G , Stamboulis E , Stefanis C , Stefanis L ((2010) ) Isolated delusional syndrome in Parkinson’s disease, Parkinsonism Relat Disord 16: , 550–552. |
[36] | Szatmari S , Illigens BM , Siepmann T , Pinter A , Takats A , Bereczki D ((2017) ) Neuropsychiatric symptoms in untreated Parkinson’s disease, Neuropsychiatr Dis Treat 13: , 815–826. |
[37] | Barrett MJ , Blair JC , Sperling SA , Smolkin ME , Druzgal TJ ((2018) ) ine symptoms and basal forebrain volume predict future psychosis in early Parkinson disease, Neurology 90,: , e1618–e1626. |
[38] | Pacchetti C , Manni R , Zangaglia R , Mancini F , Marchioni E , Tassorelli C , Terzaghi M , Ossola M , Martignoni E , Moglia A , Nappi G ((2005) ) Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson’s disease, Mov Disord 20: , 1439–1448. |
[39] | Lenka A , Hegde S , Jhunjhunwala KR , Pal PK ((2016) ) Interactions of visual hallucinations, rapid eye movement sleep behavior disorder and cognitive impairment in Parkinson’s disease: A review, Parkinsonism Relat Disord 22: , 1–8. |
[40] | Gan C , Ma K , Wang L , Si Q , Wang M , Yuan Y , Zhang K ((2021) ) Dynamic functional connectivity changes in Parkinson’s disease patients with REM sleep behavior disorder, Brain Res 1764: , 147477. |
[41] | Bejr-Kasem H , Pagonabarraga J , Martínez-Horta S , Sampedro F , Marín-Lahoz J , Horta-Barba A , Aracil-Bolaños I , Pérez-Pérez J , Ángeles Botí M , Campolongo A , Izquierdo C , Pascual-Sedano B , Gómez-Ansón B , Kulisevsky J ((2019) ) Disruption of the default mode network and its intrinsicfunctional connectivity underlies minor hallucinations inParkinson’s disease, Mov Disord 34: , 78–86. |
[42] | Lenka A , Hegde S , Arumugham AA , Singh P , Yadav R , Pal PK ((2023) ) Cognitive correlates of visual and minor hallucinations in Parkinson’s disease, Can J Neurol Sci 50: , 44–48. |
[43] | Imamura K , Wada-Isoe K , Kitayama M , Nakashima K ((2008) ) Executive dysfunction in non-demented Parkinson’s disease patients with hallucinations, Acta Neurol Scand 117: , 255–259. |
[44] | Lenka A , Hegde S , Arumugham SS , Pal PK ((2017) ) Parkinson’s pattern of cognitive impairment in patients with disease and psychosis: A critical review, Parkinsonism Relat Disord 37: , 11–18. |
[45] | Stebbins GT , Goetz GG , Carrillo MC , Bangen KJ , Turner DA , Glover GH , Gabrieli JDE ((2004) ) Altered cortical visual processing in PD with hallucinations: An fMRI study, Neurology 63: , 1409–1416. |
[46] | Lenka A , George L , Arumugham SS , Hegde S , Reddy V , Kamble N , Yadav R , Pal PK ((2017) ) Predictors of onset of psychosis in patients with Parkinson’s disease: Who gets it early? Parkinsonism Relat Disord 44: , 91–94. |
[47] | Thota N , Lenka A , George L , Hegde S , Arumugham SS , Prasad S , Stezin A , Kamble N , Yadav R , Pal PK ((2017) ) Impaired frontal lobe functions in patients with Parkinson’s disease and psychosis, Asian J Psychiatr 30: , 192–195. |
[48] | Bejr-Kasem H , Sampedro F , Marín-Lahoz J , Martínez-Horta S , Pagonabarraga J , Kulisevsky J ((2021) ) Minor hallucinations reflect early gray matter loss and predict subjective cognitive decline in Parkinson’s disease, Eur J Neurol 28: , 438–447. |
[49] | Shine JM , O’Callaghan C , Halliday GM , Lewis SJ ((2014) ) Tricks of the mind: Visual hallucinations as disorders of attention, Prog Neurobiol 116: , 58e65. |
[50] | Montagnese M , Vignando M , Ffytche D , Mehta MA ((2022) ) Cognitive and visual processing performance in Parkinson’s disease patients with vs without visual hallucinations: A meta-analysis, Cortex 146: , 161–172. |
[51] | Weintraub D , Simuni T , Caspell-Garcia C , Coffey C , Lasch S , Siderowf A , Aarsland D , Barone P , Burn D , Chahine LM , Eberling J , Espay AJ , Foster ED , Leverenz JB , Litvan I , Richard I , Troyer MD , Hawkins KA ,Parkinson’s Progression Markers Initiative ((2015) ) Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease, Mov Disord 30: , 919–927. |
[52] | Factor SA , McDonald WM , Goldstein FC ((2017) ) The role of neurotransmitters in the development of Parkinson’s disease-related psychosis, Eur J Neurol 24: , 1244–1254. |
[53] | Ballanger B , Strafella AP , van Eimeren T , Zurowski M , Rusjan PM , Houle S , Fox SH ((2010) ) Serotonin 2A receptors and visual hallucinations in Parkinson disease, Arch Neurol 67: , 416–421. |
[54] | Stahl SM ((2016) ) Parkinson’s disease psychosis as a serotonin-dopamine imbalance syndrome, CNS Spectr 21: , 355–359. |



