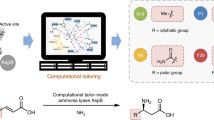
Abstract
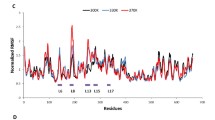
Amino ester hydrolases (AEHs) are capable of rapid synthesis of cephalexin but suffer from rapid deactivation even at low temperatures. Previous efforts to engineer AEH have generated several improved variants but have been limited in scope in part due to limitations in activity assay throughput for β-lactam synthesis reactions. Rational design of ‘whole variants’ was explored to rapidly improve AEH thermostability by mutating between 3–15% of residues. Most variants were found to be inactive due to a mutated calcium binding site, the function of which has not previously been described. Four active variants, all with improved melting temperatures, were characterized in terms of synthesis and hydrolysis activity, melting temperature, and deactivation at 25°C. Two variants were found to have improved total turnover numbers relative to the initial AEH variant; however, a clear tradeoff exists between improved stability and overall activity of each variant.






Similar content being viewed by others
References
Blum JK, Ricketts MD, Bommarius AS (2012) Improved thermostability of AEH by combining B-FIT analysis and structure-guided consensus method. J Biotechnol 160(3–4):214–221. https://doi.org/10.1016/j.jbiotec.2012.02.014
Lagerman CE, Blum JK, Rogers TA, Grover MA, Rousseau RW, Bommarius AS (2023) Total turnover number prediction of an aggregating biocatalyst: amino ester hydrolase (AEH). Chem Eng Sci 277:118804. https://doi.org/10.1016/j.ces.2023.118804
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A et al (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596(7873):583–589. https://doi.org/10.1038/s41586-021-03819-2
Porebski BT, Buckle AM (2016) Consensus protein design. Protein Eng Des Sel 29(7):245–251. https://doi.org/10.1093/protein/gzw015From NLM
Buß O, Rudat J, Ochsenreither K (2018) FoldX as Protein Engineering Tool: Better Than Random Based Approaches? Comput Struct Biotechnol J 16:25–33. https://doi.org/10.1016/j.csbj.2018.01.002
Childers MC, Daggett V (2017) Insights from molecular dynamics simulations for computational protein design. Mol Syst Des Eng 2(1):9–33. https://doi.org/10.1039/c6me00083eFrom NLM
Lippow SM, Moon TS, Basu S, Yoon SH, Li X, Chapman BA, Robison K, Lipovšek D, Prather KL (2010) Engineering enzyme specificity using computational design of a defined-sequence library. Chem Biol 17(12):1306–1315. https://doi.org/10.1016/j.chembiol.2010.10.012From NLM
Syrén P-O, Lindgren E, Hoeffken HW, Branneby C, Maurer S, Hauer B, Hult K (2010) Increased activity of enzymatic transacylation of acrylates through rational design of lipases. J Mol Catal B: Enzymatic 65(1):3–10. https://doi.org/10.1016/j.molcatb.2009.11.016
Goldenzweig A, Goldsmith M, Hill SE, Gertman O, Laurino P, Ashani Y, Dym O, Unger T, Albeck S, Prilusky J et al (2016) Automated structure- and sequence-based design of proteins for high bacterial expression and Stability. Mol Cell 63(2):337–346. https://doi.org/10.1016/j.molcel.2016.06.012
Vázquez-Figueroa E, Chaparro-Riggers J, Bommarius AS (2007) Development of a thermostable glucose dehydrogenase by a structure-guided consensus concept. ChemBioChem 8(18):2295–2301. https://doi.org/10.1002/cbic.200700500From NLM
Vázquez-Figueroa E, Yeh V, Broering JM, Chaparro-Riggers J, Bommarius AS (2008) Thermostable variants constructed via the structure-guided consensus method also show increased stability in salts solutions and homogeneous aqueous-organic media. Protein Eng Des Selection: PEDS 21 11:673–680
Rosace A, Bennett A, Oeller M, Mortensen MM, Sakhnini L, Lorenzen N, Poulsen C, Sormanni P (2023) Automated optimisation of solubility and conformational stability of antibodies and proteins. Nat Commun 14(1):1937. https://doi.org/10.1038/s41467-023-37668-6. From NLM
Blum JK, Bommarius AS (2010) Amino ester hydrolase from Xanthomonas campestris pv. Campestris, ATCC 33913 for enzymatic synthesis of ampicillin. J Mol Catal B Enzym 67(1–2):21–28. https://doi.org/10.1016/j.molcatb.2010.06.014
Polderman-Tijmes JJ, Jekel PA, de Vries EJ, van Merode AE, Floris R, van der Laan JM, Sonke T, Janssen DB (2002) Cloning, sequence analysis, and expression in Escherichia coli of the gene encoding an alpha-amino acid ester hydrolase from Acetobacter turbidans. Appl Environ Microbiol 68(1):211–218. https://doi.org/10.1128/aem.68.1.211-218.2002
Barends TRM, Polderman-Tijmes JJ, Jekel PA, Hensgens CMH, De Vries EJ, Janssen DB, Dijkstra BW (2003) The sequence and crystal structure of the α-Amino acid Ester Hydrolase fromXanthomonas citriDefine a New Family of β-Lactam Antibiotic Acylases. J Biol Chem 278(25):23076–23084. https://doi.org/10.1074/jbc.m302246200
Dam J, Schuck P (2004) Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Methods Enzymol 384:185–212. https://doi.org/10.1016/s0076-6879(04)84012-6
Kato K, Kakinuma A (1980) Dissociation and reassociation of Xanthomonas alpha-amino-acid Ester Hydrolase. Agr Biol Chem Tokyo 44(7):1663–1664. https://doi.org/10.1080/00021369.1980.10864187
Lagerman CE, Grover MA, Rousseau RW, Bommarius AS (2021) Kinetic model development for α-amino ester hydrolase (AEH)-catalyzed synthesis of β-lactam antibiotics. Chem Eng J 426:131816. https://doi.org/10.1016/j.cej.2021.131816
Bommarius AS (2023) Total Turnover Number – a Key Criterion for Process Evaluation Chemie Ingenieur Technik n/a (n/a), https://doi.org/10.1002/cite.202200177. https://doi.org/10.1002/cite.202200177 (acccessed 2023/02/21)
Rogers TA, Bommarius AS (2010) Utilizing simple biochemical measurements to Predict Lifetime output of Biocatalysts in continuous isothermal processes. Chem Eng Sci 65(6):2118–2124. https://doi.org/10.1016/j.ces.2009.12.005From NLM
Schroën CGPH, Nierstrasz VA, Kroon PJ, Bosma R, Janssen AEM, Beeftink HH, Tramper J (1999) Thermodynamically controlled synthesis of β-lactam antibiotics. Equilibrium concentrations and side-chain properties. Enzym Microb Technol 24(8):498–506. https://doi.org/10.1016/S0141-0229(98)00140-9
Acknowledgements
This work was supported by the U.S. Food and Drug Administration Center for Drug Evaluation and Research through Grant U01FD006484, which is gratefully acknowledged. C.E.L. gratefully acknowledges funding by the U.S National Science Foundation through the Graduate Research Fellowship Program (GRFP) under Grant No. DGE-1650044.
Author information
Authors and Affiliations
Contributions
C.E.L., M.A.G., R.W.R., and A.S.B. developed the concept, C.E.L., E.J., M.A.G., R.W.R., and A.S.B. developed the methodology, C.E.L., E.J., M.A.G., R.W.R., and A.S.B. conducted the investigation, M.A.G., R.W.R., A.S.B. acquired the funding for the project, M.A.G., R.W.R., and A.S.B. administered the project, C.E.L., E.J., M.A.G., R.W.R., and A.S.B. wrote the original draft, and C.E.L., E.J., M.A.G., R.W.R., and A.S.B. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lagerman, C.E., Joe, E.A., Grover, M.A. et al. Improvement of α-amino Ester Hydrolase Stability via Computational Protein Design. Protein J 42, 675–684 (2023). https://doi.org/10.1007/s10930-023-10155-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-023-10155-z