Abstract
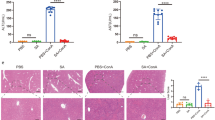
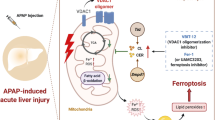
Hepatic sinusoidal obstruction syndrome (HSOS) is a death-dealing liver disease with a fatality rate of up to 67%. In the study present, we explored the efficacy of andrographolide (Andro), a diterpene lactone from Andrographis Herba, in ameliorating the monocrotaline (MCT)-induced HSOS and the underlying mechanism. The alleviation of Andro on MCT-induced rats HSOS was proved by biochemical index detection, electron microscope observation, and liver histological evaluation. Detection of hepatic ATP content, mitochondrial DNA (mtDNA) copy number, and protein expression of nuclear respiratory factor-1 (NRF1) and peroxisome proliferator–activated receptor gamma coactivator 1 alpha (PPARGC1A) demonstrated that Andro strengthened mitochondrial biogenesis in livers from MCT-treated rats. Chromatin immunoprecipitation assay exhibited that Andro enhanced the occupation of nuclear factor erythroid 2-related factor 2 (NFE2L2, also known as NRF2) in the promoter regions of both PPARGC1A and NRF1. Andro also activated the NRF2-dependent anti-oxidative response and alleviated liver oxidative injury. In Nrf2 knock-out mice, MCT induced more severe liver damage, and Andro showed no alleviation in it. Furthermore, the Andro-activated mitochondrial biogenesis and anti-oxidative response were reduced in Nrf2 knock-out mice. Contrastingly, knocking out Kelch-like ECH-associated protein 1 (Keap1), a NRF2 repressor, reduced MCT-induced liver damage. Results from co-immunoprecipitation, molecular docking analysis, biotin-Andro pull-down, cellular thermal shift assay, and surface plasmon resonance assay showed that Andro hindered the NRF2-KEAP1 interaction via directly binding to KEAP1. In conclusion, our results revealed that NRF2-dependent liver mitochondrial biogenesis and anti-oxidative response were essential for the Andro-provided alleviation of the MCT-induced HSOS.
Graphical abstract
Graphical Headlights:
1. Andro alleviated MCT-induced HSOS via activating antioxidative response and promoting mitochondrial biogenesis.
2. Andro-activated antioxidative response and mitochondrial biogenesis were NRF2-dependent.
3. Andro activated NRF2 via binding to KEAP1.









Similar content being viewed by others
Data availability
Data and materials used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- ALT/AST:
-
Alanine/aspartate aminotransferase
- Andro:
-
Andrographolide
- ARE:
-
Antioxidant-responsive element
- ATP:
-
Adenosine triphosphate
- CETSA:
-
Cellular thermal shift assay
- ChIP:
-
Chromatin immunoprecipitation
- CoIP:
-
Co-immunoprecipitation
- COX4I1:
-
Cytochrome c oxidase subunit 4I1
- CYCS:
-
Cytochrome C
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- GCLC:
-
Catalytic subunit of glutamate-cysteine ligase
- GCLM:
-
Modifier subunit of glutamate-cysteine ligase
- GSR:
-
Glutathione-disulfide reductase
- HHSEC:
-
Human hepatic sinusoidal endothelial cell
- HO-1:
-
Hemeoxygenase1
- HSCT:
-
Hematopoietic stem cell transplantation
- HSOS:
-
Hepatic sinusoidal obstruction syndrome
- H2DCFDA:
-
2′-7′-Dichlorodihydro-fluorescein diacetate
- KEAP1:
-
Kelch-like ECH-associated protein 1
- LSECs:
-
Liver sinusoidal endothelial cells
- MCT:
-
Monocrotaline
- MDA:
-
Malondialdehyde
- MMP9:
-
Matrix metalloproteinase 9
- mtDNA:
-
Mitochondrial DNA
- nDNA:
-
Nuclear DNA
- NRF1:
-
Nuclear respiratory factor 1
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- PBS:
-
Phosphate-buffered saline
- PPARGC1A:
-
Peroxisome proliferator–activated receptor gamma coactivator 1α
- ROS:
-
Reactive oxygen species
- SEM:
-
Standard error of the mean
- SOD:
-
Superoxide dismutase
- TFAM:
-
Mitochondrial transcription factor A
- TOM20:
-
Outer mitochondrial membrane 20
- TRX1:
-
Thioredoxin 1
- TRXR1:
-
Thioredoxin reductase 1
References
Anzell AR, Maizy R, Przyklenk K, Sanderson TH. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol. 2018;55(3):2547–64. https://doi.org/10.1007/s12035-017-0503-9.
Athale J, Ulrich A, MacGarvey NC, Bartz RR, Welty-Wolf KE, Suliman HB, et al. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med. 2012;53(8):1584–94. https://doi.org/10.1016/j.freeradbiomed.2012.08.009.
Bi JB, Zhang J, Ren YF, Du ZQ, Li QS, Wang Y, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296–306. https://doi.org/10.1016/j.redox.2018.10.019.
Cabrera D, Wree A, Povero D, Solis N, Hernandez A, Pizarro M, et al. Andrographolide ameliorates inflammation and fibrogenesis and attenuates inflammasome activation in experimental non-alcoholic steatohepatitis. Sci Rep. 2017;7(1):3491. https://doi.org/10.1038/s41598-017-03675-z.
Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–9. https://doi.org/10.1016/j.cell.2010.02.026.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53(2):138–45. https://doi.org/10.1038/bmt.2017.161.
Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transplant. 2016;22(3):400–9. https://doi.org/10.1016/j.bbmt.2015.09.024.
Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125(3):882–90. https://doi.org/10.1016/s0016-5085(03)01056-4.
Ding LL, Li JM, Song BL, Xiao X, Huang WD, Zhang BF, et al. Andrographolide prevents high-fat diet-induced obesity in C57BL/6 mice by suppressing the sterol regulatory element-binding protein pathway. J Pharmacol Exp Ther. 2014;351(2):474–83. https://doi.org/10.1124/jpet.114.217968.
Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88(Pt B):179–88. https://doi.org/10.1016/j.freeradbiomed.2015.04.036.
Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, et al. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol. 2017;108(Pt A):339–50. https://doi.org/10.1016/j.fct.2017.08.020.
Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4(4):332–46. https://doi.org/10.1016/j.jceh.2014.10.002.
Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh QA, Allzrag A, et al. Andrographis paniculata (Burm. f.) Wall. ex Nees: an updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life (Basel). 2021;11(4):348. https://doi.org/10.3390/life11040348.
Hu LQ, Magesh S, Chen L, Wang LL, Lewis TA, Chen Y, et al. Discovery of a small-molecule inhibitor and cellular probe of Keap1-Nrf2 protein-protein interaction. Bioorg Med Chem Lett. 2013;23(10):3039–43. https://doi.org/10.1016/j.bmcl.2013.03.013.
Huang ZL, Sheng YC, Chen MW, Hao ZX, Hu FF, Ji LL. Liquiritigenin and liquiritin alleviated MCT-induced HSOS by activating Nrf2 antioxidative defense system. Toxicol Appl Pharmacol. 2018;355:18–27. https://doi.org/10.1016/j.taap.2018.06.014.
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. https://doi.org/10.1101/gad.13.1.76.
Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundback T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9(9):2100–22. https://doi.org/10.1038/nprot.2014.138.
Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12–23. https://doi.org/10.1016/j.freeradbiomed.2015.03.035.
Jing XQ, Zhang JQ, Huang ZL, Sheng YC, Ji LL. The involvement of Nrf2 antioxidant signalling pathway in the protection of monocrotaline-induced hepatic sinusoidal obstruction syndrome in rats by (+)-catechin hydrate. Free Radic Res. 2018;52(4):402–14. https://doi.org/10.1080/10715762.2018.1437914.
Kwaan HC, Samama MM. The significance of endothelial heterogeneity in thrombosis and hemostasis. Semin Thromb Hemost. 2010;36(3):286–300. https://doi.org/10.1055/s-0030-1253451.
Lee TY, Chang HH, Wen CK, Huang TH, Chang YS. Modulation of thioacetamide-induced hepatic inflammations, angiogenesis and fibrosis by andrographolide in mice. J Ethnopharmacol. 2014;158 Pt A:423–30. https://doi.org/10.1016/j.jep.2014.10.056.
Li PA, Hou X, Hao S. Mitochondrial biogenesis in neurodegeneration. J Neurosci Res. 2017;95(10):2025–9. https://doi.org/10.1002/jnr.24042.
Lin LT, Li R, Cai MY, Huang JJ, Huang WS, Guo YJ, et al. Andrographolide Ameliorates liver fibrosis in mice: involvement of TLR4/NF-kappaB and TGF-beta1/Smad2 signaling pathways. Oxid Med Cell Longev. 2018;2018:7808656. https://doi.org/10.1155/2018/7808656.
Martinez MD, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341(6141):84–7. https://doi.org/10.1126/science.1233606.
Mussard E, Cesaro A, Lespessailles E, Legrain B, Berteina-Raboin S, Toumi H. Andrographolide, a natural antioxidant: an update. Antioxidants (Basel). 2019;8(12):571. https://doi.org/10.3390/antiox8120571.
Nishinaka T, Miura T, Shimizu K, Terada T. Identification and characterization of functional antioxidant response elements in the promoter of the aldo-keto reductase AKR1B10 gene. Chem Biol Interact. 2017;276:160–6. https://doi.org/10.1016/j.cbi.2017.02.008.
Pan CW, Yang SX, Pan ZZ, Zheng B, Wang JZ, Lu GR, et al. Andrographolide ameliorates d-galactosamine/lipopolysaccharide-induced acute liver injury by activating Nrf2 signaling pathway. Oncotarget. 2017;8(25):41202–10. https://doi.org/10.18632/oncotarget.17149.
Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266(18):11632–9.
Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23(9):459–66. https://doi.org/10.1016/j.tem.2012.06.006.
Song Y, Wu XQ, Yang D, Fang F, Meng LS, Liu Y, et al. Protective effect of andrographolide on alleviating chronic alcoholic liver disease in mice by inhibiting nuclear factor kappa b and tumor necrosis factor alpha activation. J Med Food. 2020;23(4):409–15. https://doi.org/10.1089/jmf.2019.4471.
Steelandt J, Bocquet F, Cordonnier AL, De Courtivron C, Fusier I, Paubel P. Defibrotide in severe sinusoidal obstruction syndrome: medicine and economic issues. Biol Blood Marrow Transplant. 2017;23(2):347–56. https://doi.org/10.1016/j.bbmt.2016.11.020.
Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev. 2016;68(1):20–48. https://doi.org/10.1124/pr.115.011502.
Tiao MM, Lin TK, Chen JB, Liou CW, Wang PW, Huang CC, et al. Dexamethasone decreases cholestatic liver injury via inhibition of intrinsic pathway with simultaneous enhancement of mitochondrial biogenesis. Steroids. 2011;76(7):660–6. https://doi.org/10.1016/j.steroids.2011.03.002.
Valla DC, Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol. 2016;40(4):378–85. https://doi.org/10.1016/j.clinre.2016.01.006.
Vomund S, Schafer A, Parnham MJ, Brune B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18(12):2772. https://doi.org/10.3390/ijms18122772.
Wei MJ, Zheng ZY, Shi L, Jin Y, Ji LL. Natural polyphenol chlorogenic acid protects against acetaminophen-induced hepatotoxicity by activating ERK/Nrf2 antioxidative pathway. Toxicol Sci. 2018;162(1):99–112. https://doi.org/10.1093/toxsci/kfx230.
Xie SY, Deng W, Chen JJ, Wu QQ, Li HJ, Wang J, et al. Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. 2020;16(1):12–26. https://doi.org/10.7150/ijbs.37269.
Yan HY, Huang ZL, Bai QY, Sheng YC, Hao ZX, Wang ZT, et al. Natural product andrographolide alleviated APAP-induced liver fibrosis by activating Nrf2 antioxidant pathway. Toxicology. 2018;396–397:1–12. https://doi.org/10.1016/j.tox.2018.01.007.
Yang XQ, Ye J, Li X, Li Q, Song YH. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J Gastroenterol. 2019;25(28):3753–63. https://doi.org/10.3748/wjg.v25.i28.3753.
Ye JF, Zhu H, Zhou ZF, Xiong RB, Wang XW, Su LX, et al. Protective mechanism of andrographolide against carbon tetrachloride-induced acute liver injury in mice. Biol Pharm Bull. 2011;34(11):1666–70. https://doi.org/10.1248/bpb.34.1666.
Zhang YK, Wu KC, Klaassen CD. Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. Plos One. 2013;8(3):e59122. https://doi.org/10.1371/journal.pone.0059122.
Funding
This work was financially supported by the “Young Qihuang Scholar” and the Shanghai Excellent Academic Leaders Program (23XD1404000) for Lili Ji, the National Natural Science Foundation of China (82104509), and the Shanghai Pujiang Program (22PJ1412900) for Zhenlin Huang.
Author information
Authors and Affiliations
Contributions
Zhenlin Huang and Lili Ji conceptualized this study and designed the research. Zhenlin Huang, Zeqi Wu, Jingnan Zhang, Keke Wang, Qing Zhao, Minwei Chen, Shihao Yan, and Qian Guo conducted the experiments and analyzed the data. Zhenlin Huang drafted the manuscript. Yun Ma and Lili Ji revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures were performed in accordance with the guidelines of the Experimental Animal Ethical Committee of the Shanghai University of Traditional Chinese Medicine (Approval Number: PZSHUTCM200403010).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Wu, Z., Zhang, J. et al. Andrographolide attenuated MCT-induced HSOS via regulating NRF2-initiated mitochondrial biogenesis and antioxidant response. Cell Biol Toxicol 39, 3269–3285 (2023). https://doi.org/10.1007/s10565-023-09832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-023-09832-7